In this blog, we break down the difference between conventional and digital surface plasmon resonance (SPR) systems to help you select the right solution for your lab.
Introduction
Biomolecular interactions are often at the heart of therapeutic discovery as they promote the regulation and execution of most biological processes in the body. Thus, early drug discovery relies heavily on the characterization of these interactions to validate new targets and observe their binding to new drugs.
Surface plasmon resonance (SPR) is a powerful technique for observing biomolecular interactions due to its ability to quantitatively measure real-time interactions without the need for labels or markers. While SPR is a critical tool in the discovery and development of biologics, conventional systems have often been regarded as too technically challenging or resource-consuming to adopt in the early stages of the drug discovery workflow. To help you choose the right label-free analytical system for your lab, this article reviews the differences between conventional and digital SPR systems.
Blog overview
- SPR: A gold standard for label-free analysis
- Conventional SPR systems
- Digital SPR systems
- Comparing SPR workflows
SPR: A gold standard for label-free analysis
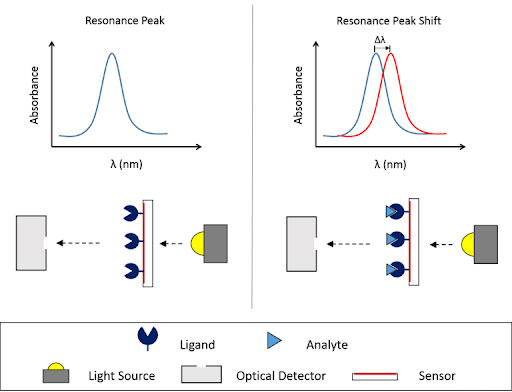
SPR is an optical phenomenon that occurs when an incident light is directed at a metal surface and stimulates the electrons of the metal’s conductive band, generating surface electromagnetic waves called evanescent waves. SPR biosensors are able to exploit these waves to measure minute changes in the refractive index of the medium near the metal surface.
SPR instruments are made up of an optical measurement system to measure the changes in refractive index in real-time, a fluid handling system for sample delivery, and a sensor with a functionalized surface. During an experiment, one binding partner is typically immobilized to the sensor surface (the ligand), while the other binding partner (the analyte) is flowed across the surface in solution. If binding occurs, the mass change on the surface alters the refractive index of the medium and is measured in real time, allowing the user to calculate key binding constants such as the rate of association and dissociation, as well as affinity.
SPR biosensors have become a standard tool in the biopharmaceutical industry due to their ability to characterize a wide range of interaction types and provide information-rich data. As such, they are commonly leveraged in drug discovery and development to quickly advance new therapeutic candidates to market.
Conventional SPR systems
Conventional label-free instruments rely on complex networks of pumps, valves, tubing and flow cells to ensure the delivery of sample to and from the sensor surface. They typically require large volumes of samples and buffers, ranging anywhere between 100-500µl, to ensure adequate contact between the sample and sensor surface and to help account for sample dispersion driven by pressure driven flow.
Recent years have seen increasingly complex approaches being applied in biologics development in the continual push to condense the development cycle and reduce the high attrition rates. While SPR systems are known to be far less demanding in their sample requirements compared to more common techniques, it is more critical now than ever for discovery tools such as SPR to provide a greater economies of scale while enabling deeper characterization of novel leads earlier in the development process.
In addition, conventional SPR systems suffer from limited throughput, usability and output that ultimately slows down the discovery process. The complex hardware of SPR systems make them prone to fluidic clogging and degradation, particularly when using non-homogenous or crude samples. Usage is also limited when working with high or low affinity binders that require copious volumes of sample and reagents to accurately determine the kinetic parameters of an interaction. The technology employed in conventional SPR systems also tends to make them more sensitive to external interferences and prone to error, further making them challenging to use. These are just some of the many reasons for why SPR has often been regarded as a challenging and inflexible technique.
At Nicoya, we integrate localized SPR (LSPR) technology in all our systems to make them more robust and cost-effective. Learn more about the advantages of localized SPR technology from our article comparing LSPR to conventional SPR.
Digital SPR systems
At Nicoya, we developed Alto, the first system to digitalize SPR by integrating digital microfluidics (DMF), an advanced fluid-handling technology that precisely manipulates nano-liter sized droplets to perform complex laboratory protocols. Alto is a 16-channel benchtop system that provides high-throughput affinity and kinetics analysis, allowing for simultaneous analysis of up to 8 unique ligands in a single run.
While the core analysis closely resembles that of conventional SPR, the integration of DMF allows for the same protocols to be performed on Alto with significantly lower volumes and in an error-free fashion through the automation of reagent preparation and serial dilutions. Furthermore, the technology allows for the miniaturization of the overall assay, thus giving Alto an economical lab footprint and making it a robust system that requires minimal maintenance. By coupling SPR biosensors directly onto a DMF-based disposable cartridge, Alto provides a fluidics-free instrument which uniquely offers the ability to analyze complex samples that would otherwise pose a challenge with conventional systems.
Comparing SPR workflows
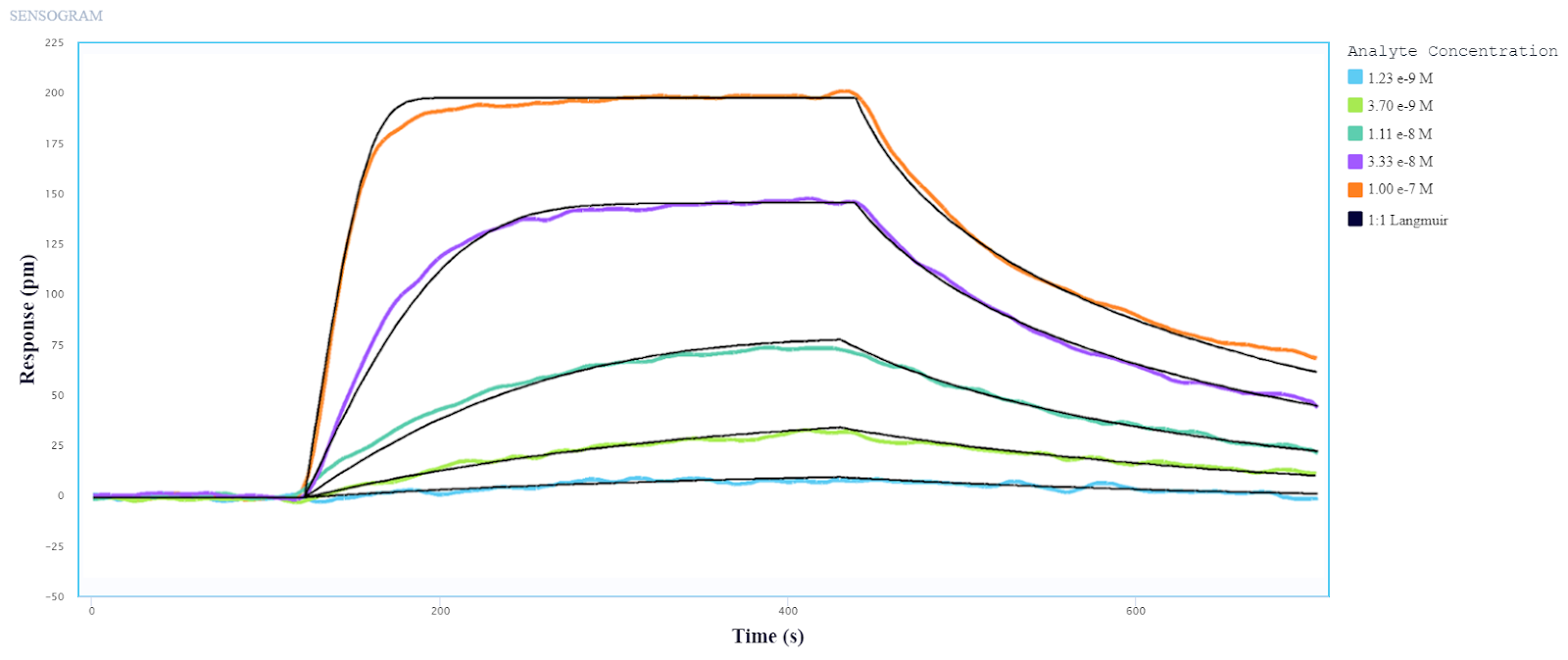
In selecting a system that will accelerate your drug discovery, it is important to consider how it will optimize the overall cost and time of your workflow. We recently conducted a study comparing the performance of a conventional flow-based SPR system to Alto in the selection of antibodies from Human Combinatorial Antibody Libraries (HuCAL).
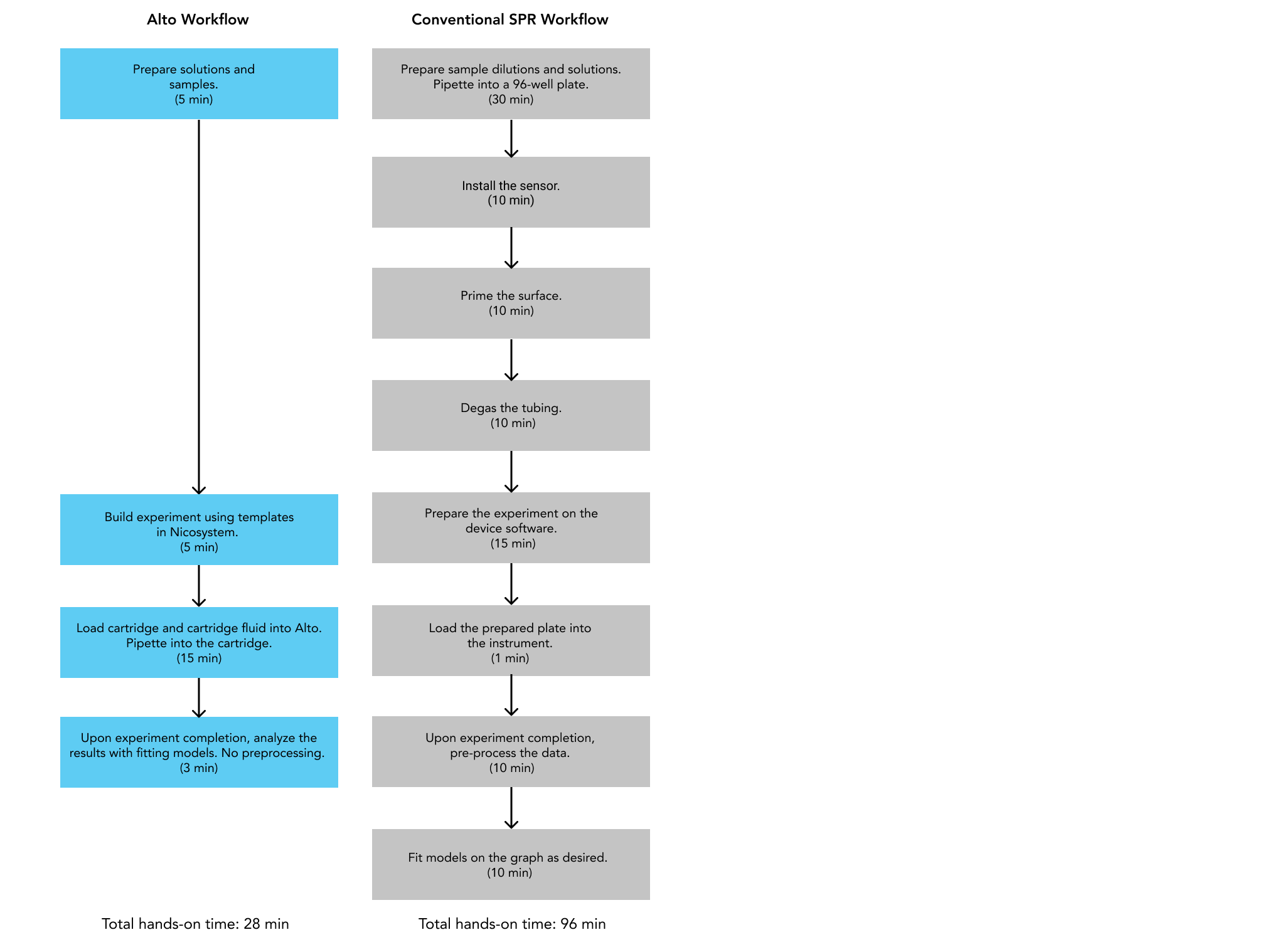
In this experiment, performing the same assay on Alto successfully saved the operator over an hour of time compared to its conventional counterpart, reducing the required hands-on time by 70%. By using just a fraction of the total sample, Alto also measured the kinetics and affinity of the HuCAL-GFP binding system with equivalent accuracy and comparable standard errors, as shown in Figure 4.
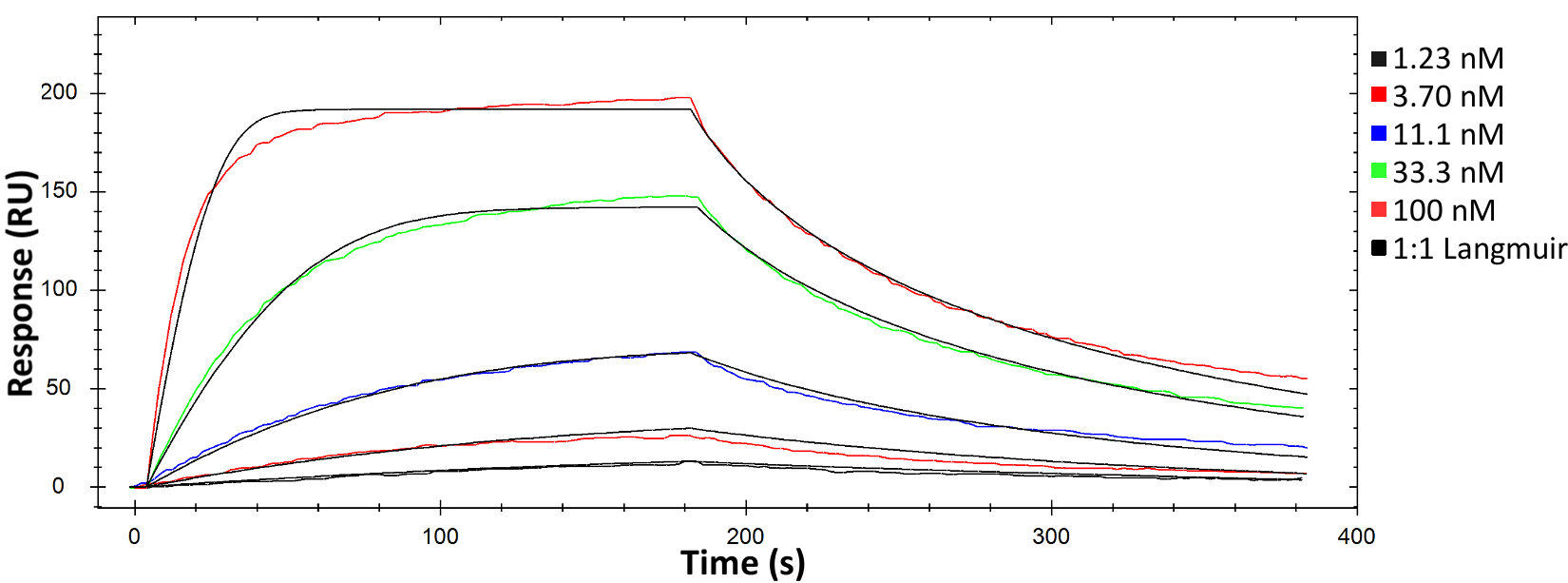
To view the the full experiment, read our full application note on Characterization of Biomolecular Interactions using Alto: Comparison to Conventional SPR.
As a powerful accelerant in our understanding of disease, SPR is a critical tool in the discovery and development of novel biological drugs. In our mission to improve human life by helping scientists succeed, we developed Alto to overcome the common challenges of using SPR to provide a more cost-effective and efficient solution for biologics discovery and development. To learn more about how Alto’s ease-of-use and low sample volumes can accelerate your time to discovery, contact our team today!