Accuracy, precision and reproducibility are the three most important principles in scientific research. When conducting an experiment using any technique and under any condition, these three principles will be the dividing lines between an accepted manuscript or a rejected one. Nicoya’s OpenSPR instrument measures binding kinetics in real-time and in a label-free manner. However, physical measurement of binding kinetics is only one piece of the puzzle; SPR data analysis and interpretation of your results are what will provide the subsurface information with your research project needs. In this blog, we provide helpful insight into minimizing unwanted artefact, analyzing SPR data and teach you how to make your SPR curves precise, accurate and reproducible. To preface our points of discussion, below is a list of some parameters, phenomena and areas of interest for optimal SPR data:
- Design and optimization
- Non-specific binding
- Mass transfer
- Fit models
Design and Optimization
Robust assay design is important when working with any piece of analytical instrumentation. Having a strong design will ensure accurate, precise and reproducible SPR data. With that said, to achieve publication-quality SPR data, assay design and optimization are the first critical control points to mitigate lengthy discussions between you and a reviewer. Things to consider when designing and optimizing SPR experiments to maximize the impact potential of the assay may include (but are not restricted to) proper positive and negative controls, running buffer pH, flow rate(s), isoelectric points (pI), purity levels, solubility, regeneration conditions, mass transfer limitations, ligand concentrations and analyte concentrations (to name a few!).
For time purposes, we’ll use analyte concentration optimization as an example; assuming you have an expected KD value of your interactants. It’s necessary to choose five concentrations that reside within a range of 0.1 – 10x the expected KD of your interactants. Notably, if this guideline is not accounted for during design or optimization, lost time will accumulate. Using this as an example, you may begin to see the importance of robust SPR assay design and the value of comprehensive customer support. The following information will help you and your team with adequate assay design and optimization.
Non-Specific Binding
Non-specific binding (NSB) is the phenomena observed when the analyte of interest interacts with the sensor surface via hydrophobic, charge or hydrogen forces and causes unwanted background signal. NSB can be a challenging phenomenon to overcome if you lack mitigation knowledge before starting SPR experiments. The key to good vs. bad SPR data (in terms of NSB) is to ensure that the non-specific background signal does not occlude the expected specific signal. Non-specific binding tests are always encouraged to be conducted before the actual collection of SPR data and are quite simple to run. NSB tests will allow you to gauge the amount of expected NSB from your analyte to your sensor and in turn, enable effective sensor chemistry selection. It is important to note that if the level of specific binding sufficiently overrides the level of NSB, NSB reduction strategies may not even be necessary. With that said, there are four extremely useful tips to help eliminate NSB from your binding system and make your SPR data a treat for any reviewer:
- Adjust pH to the isoelectric point of your analyte
- Add NMT 1% BSA in your analyte running buffer
- Use Tween 20 to eradicate hydrophobic interactions with the sensor
- Add more salt to your running buffers to disrupt charge interactions with the sensor
Mass Transfer
When one interactant resides in solution, and the other interactant remains tethered to a surface, the solution-interactant must “come out” of the bulk in order to interact with the immobilized partner. However, if the diffusion rate is slower than the association rate, mass transfer effects can be observed in the data; this can hold true for fast binding interactions. But how do you know if you have a mass transport limited binding system? Well, a quick way to determine if you have a mass transfer limited system is to inject the analyte at a few different flow rates; if the on-rate decreases at lower flow rates, the interaction can be classified as mass transport limited. But how do we stop this from happening? There are three simple ways to either mitigate or address mass transport from negatively impacting your SPR data:
- Use a higher flow rate (addressed above)
- Lower ligand density so less analyte needs to diffuse out of solution
- Use a mass-transport corrected fit model (Tip: always optimize before you switch fit models)
Fit Models
SPR data analysis is a very simple thing to overlook. It is not uncommon for researchers to fit their measured curves with the simplest binding model (a 1:1 global fit), only to realize the fit isn’t ideal and begin to “shop” for a better fit. Although this makes sense when you want to buy a pair of jeans, the same does not hold true in SPR data analysis. It is so important to understand why your data doesn’t fit well instead of trying to figure out how you can make it fit better with software. Keeping in mind that SPR data analysis software has more fit models than the 1:1 model for a reason, the only time you should be using these alternative models is when you have adequate experimental evidence suggesting an alternative binding model is present in your binding system. To bring this article full circle, adequate controls, experimental design and optimization is the best recipe to produce rockstar SPR data. However, there are cases where a 1:1 model should not be used:
- Mass transport limited binding systems
- Bivalent binding systems
- Modulating interactions (ie. cooperative binding)
In summary, it is imperative to make sure your controls, experimental design and optimization are robust when conducting kinetic experiments with SPR. As we mentioned earlier, a lot of the unwanted artefact found in SPR experiments can be mitigated proactively with robust experimental design. Nicoya’s customer support team is composed of experienced SPR experts that will be there every step of the way, from designing your SPR assay to publication.
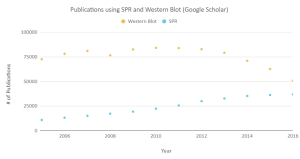
OpenSPR is a user-friendly and low maintenance benchtop SPR solution that is currently being used by hundreds of researchers. With access to SPR technology on your own lab bench, you can get the high-quality data you need to accelerate your research and publish successfully. SPR is necessary not only for publications but for the advancement of many fields of medicine and medical research as can be seen below with the significant increase in publications that rely on SPR data.