Flow cytometers are excellent tools for the modern cell biologist and immunologist. They can provide a wide range of information which help researchers discern various cellular physical and chemical characteristics. Being an extremely versatile instrument, there are many applications for flow cytometry including cell counting, sorting, viability, apoptosis detection, membrane potential and even binding assays. In this article, we discuss applications for flow cytometry and demonstrate how you can use benchtop surface plasmon resonance to complement your flow cytometry work to get the kinetic data needed to publish faster.
How does flow cytometry work?
Flow cytometers are optics-based instruments that operate by way of a fluidics system, laser, a series of optical filters and a detector. An injection of fluorescently-labeled cell suspension is made into the instrument and cells pass through a fluidic bottleneck which funnels the cells to move into single file. Cells then pass towards the measurement area where a laser illuminates each cell and based on the physical and/or chemical features within the cell, light is scattered. Fluorophores on the cell will also become excited and emit towards the detector. After cells are interrogated with the laser, they will either go to waste or become sorted and passed into collection tubes for downstream assays.
What information can I gain from flow cytometers?
Flow cytometers can carry out an array of quantitative measurements including cell counting, sorting, viability, apoptosis detection, membrane potential and apparent affinity measurements. Importantly, biotherapeutics have become a major area of study for treatment of cancers, immune disorders and more. Notably, candidate biotherapeutics that target cell surface receptors on immune cells, have unique binding characteristics that are very important parameters to quantitate. Interestingly, there are very common assays that can be carried out with flow cytometers that can determine apparent affinities from biotherapeutic-receptor binding events.
Receptor Occupancy (RO) Assay
A receptor occupancy assay is a high-throughput method whereby binding affinity between a candidate therapeutic (or biotherapeutic) can be measured. Notably, an RO assay involves two crucial “sub-assays”. As part of the first sub-assay, cells bound by a fluorescently-labeled, non-competing antibody are injected into the flow cytometer and data is collected. Subsequently, conjugated cells and the target antibody are injected into the instrument and upon generation of a standard curve, total expression of the surface receptor can be quantitated.
In the second sub-assay, cells should be conjugated with unlabeled target antibody but some of the target antibody should be labeled separately. Cells conjugated with unlabeled target antibody are injected and data is recorded. Subsequently, an injection of the labeled target antibody is made and at this point, only the free receptors will bind the labeled antibody. Using a standard curve, it is possible to determine the number of free receptors.
Notably, when these two sub-assays are combined, it is possible to calculate a value known as receptor occupancy. Subsequently, this value can be converted into an apparent affinity value and if the experiments are carried out over extended time periods, indirect kinetic values can be calculated.
Complement Flow Cytometry with Surface Plasmon Resonance
Although indirect measurement of kinetic values is a good starting point to understand the binding events taking place between your biotherapeutics and receptors, direct measurements are significantly more comprehensive and accurate. Upon completion of your high-throughput screening with flow cytometry, you have identified hits and derived apparent affinities. To take your research closer to publication, Surface Plasmon Resonance (SPR) will provide a simplistic, quantitative and label-free method to directly measure kon, koff and KD between your candidates and surface receptors.
Keeping in mind that flow cytometry is carried out ex vivo with intact cells and purified antibody, it is recommended to purify or obtain purified receptor to immobilize on a sensor chip for in vitro, SPR kinetic assays. When used in tandem with flow cytometry, SPR is an outstanding complement to strengthen your publication.
With that said, there are a few techniques that can provide quantitative binding data including ITC, MST and BLI as well as fluorescent techniques. However, these technologies typically use a lot of sample, require labels, do not provide enough information and/or require large capital expenditure. However, Surface Plasmon Resonance (SPR) has been the gold standard for obtaining quantitative binding kinetics data for a number of decades. Importantly, OpenSPR is a user-friendly and low maintenance benchtop SPR solution that is currently being used by hundreds of researchers. With access to SPR technology on your own lab bench, you can get the high-quality data you need to accelerate your research and publish faster.
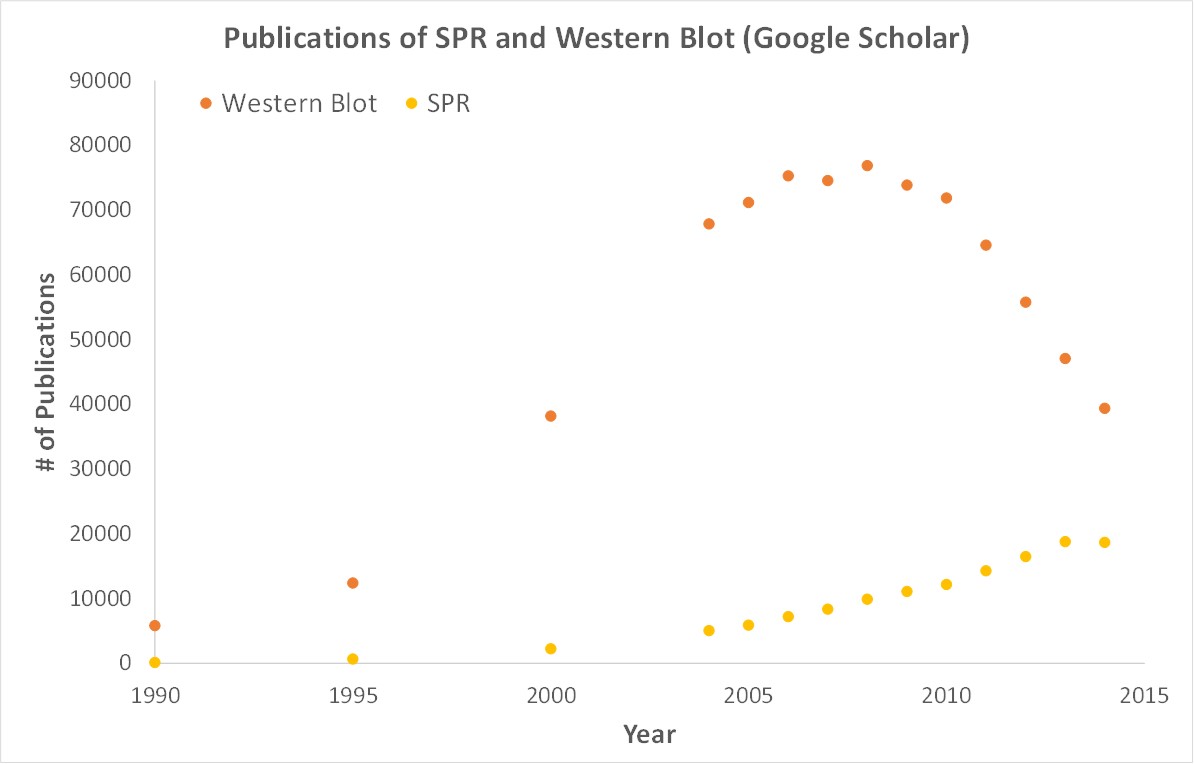
SPR is necessary not only for publications but for the advancement of many fields of medicine and medical research as can be seen below with the significant increase in publications that rely on SPR data.