Explore how OpenSPR is advancing disease research in over 200 peer-reviewed publications
Affordability and ease of use have made OpenSPR® a powerful tool in labs worldwide, providing high-quality binding data to hundreds of researchers publishing in drug discovery, disease research, diagnostics and more.
In their recent review publication, researchers Eliza Hanson and Dr. Rebecca Whelan from The University of Kansas summarize OpenSPR peer-reviewed publications between 2016 and 2022. They highlight the range of biomolecular analytes and interactions that have been investigated using the platform, provide an overview of the most common applications of OpenSPR, and point out some representative research that highlights the flexibility and utility of the instrument.
Table of contents
- OpenSPR’s groundbreaking technology
- Applications of OpenSPR in the past decade
- Meet Whelan and Hanson
- Conclusion
A look at our OpenSPR-XT and OpenSPR instruments.
1. OpenSPR’s groundbreaking technology
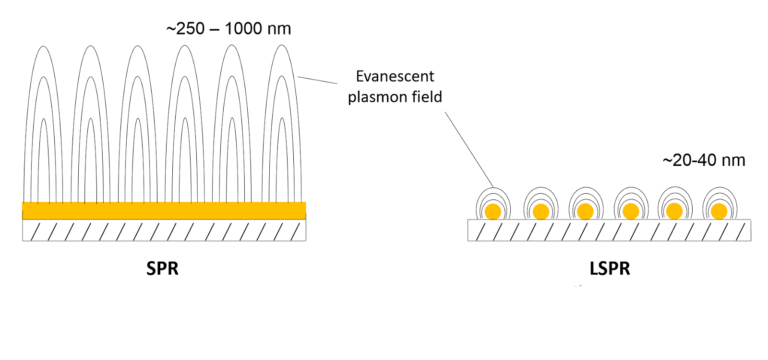
OpenSPR is the world’s only benchtop surface plasmon resonance (SPR) instrument, providing real-time, high-quality, label-free interaction analysis for a fraction of the cost of other existing solutions. The unique nanotechnology-based sensors produce a localized SPR (LSPR) phenomenon, and along with the intuitive software interface, makes the instrument easy to use and affordable, while working to generate publication-quality data.
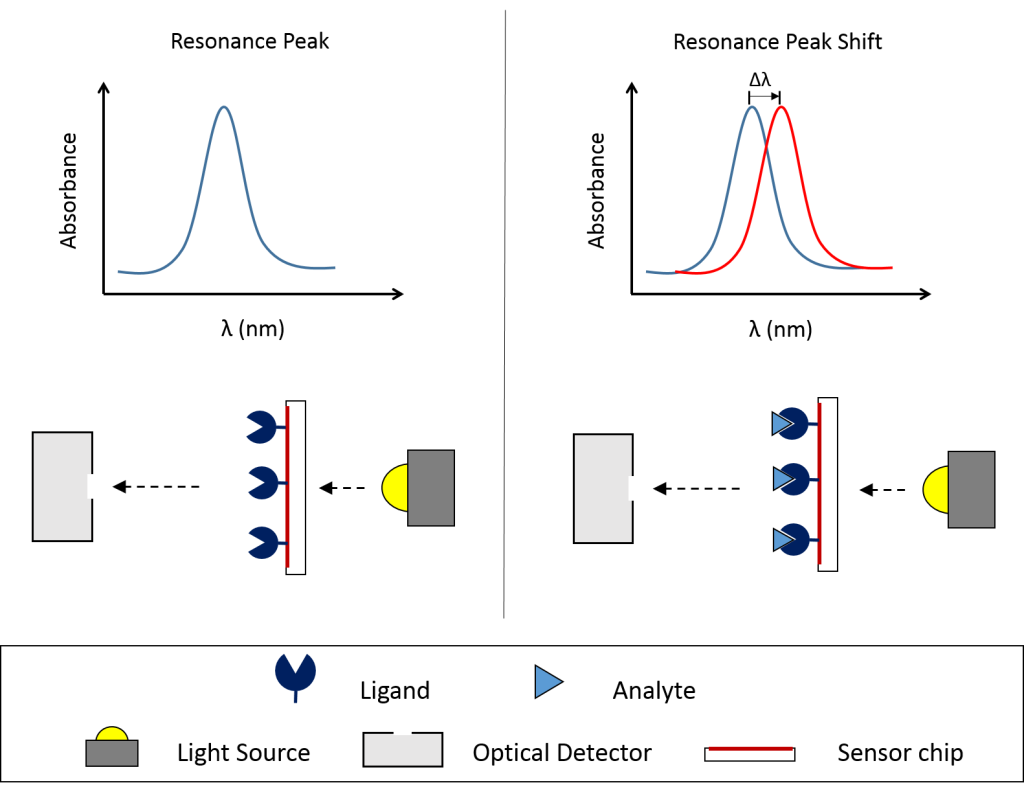
The use of LSPR allows OpenSPR to require less maintenance than traditional SPR solutions. and it is more susceptible to binding while being less susceptible to bulk and vibrational & mechanical noise. A binding affinity and kinetics assay on the instrument consists of the immobilization of a binding partner (ligand) followed by the injection of another binding partner (analyte). The repetition of analyte injection sends corresponding signals to the sensor chip and allows the generation of data throughout the experiment. The average end signal from each injection plotted against concentration can calculate KD, or be placed into the analysis software to calculate kon and koff.
Owing to its user-friendliness and versatility, OpenSPR is currently used in more than 500 research labs worldwide and has been cited in over 315 publications. This includes research in therapeutic areas ranging from oncology, immunology and infectious disease as well as applications compatible with proteins/peptides, antibodies, nucleic acids, lipids, small molecules (application dependent), adeno-associated viruses (AAV), virus-like particles (VLPs), hormones/cytokines, and crude samples.
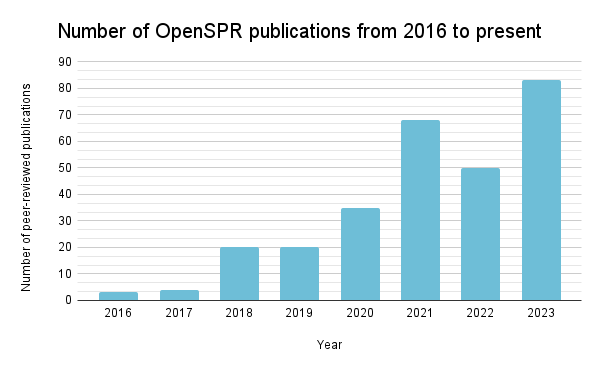
A look at OpenSPR publications from 2016 to the present.
2. Applications of OpenSPR in the past decade
A recent review article covered an exhaustive survey of 200 peer-reviewed publications between 2016 and 2022 that utilized OpenSPR, showcasing its application across a broad spectrum of biomolecular studies. It has been employed in research targeting critical health issues like Alzheimer’s disease, lung cancer, and COVID-19, demonstrating OpenSPR’s versatility and utility in biomolecular analysis.
This publication finds that the most common interaction investigated was protein-protein, followed by protein-small molecule. While most papers used OpenSPR to characterize two binding partners, a few looked into characterizing the interaction between more, such as a collaborative protein–protein-nucleic acid binding.
Papers using the OpenSPR platform were also found to investigate a wide variety of targets, the majority being protein, followed by small molecules, peptides, nucleic acid, carbohydrates, lipids, nanomaterials, polymers, and viruses. The instrument was also used to measure the kinetics of potential affinity reagents, such as a paper where an aptamer was selected by Lu and coworkers to “detect di(2-ethylhexyl) phthalate (DEHP), a plasticizer commonly used as an additive in food packaging with toxic effects as an androgen antagonist,” demonstrating the wide range of supported assays (Hanson & Whelan, 2023).
OpenSPR has been compared favourably with other commercial SPR systems, highlighting its competitive performance in terms of sensitivity, range of analytes measurable, and user-friendliness at a lower cost. Beyond OpenSPR and Nicoya’s Alto™, a high-throughput SPR platform, the future for benchtop SPR instruments looks bright, offering new capabilities and further simplifying sample handling for complex assays.
3. Meet Whelan and Hanson
We sat down with the authors of the review, Professor Rebecca Whelan and graduate student Eliza Hanson from the University of Kansas to learn more about their lab, research, and experience with OpenSPR.
“Tell us a bit about your lab and team at the University of Kansas.”
RJW: The Whelan Lab is a collection of bioanalytical chemists who work on important questions in human health. The lab recently relocated to the University of Kansas (KU) where we are fortunate to be affiliated with the Ralph N. Adams Institute for Bioanalytical Chemistry and the KU Cancer Center. One thing that makes the lab special is our culture of collaboration and mutual support. We collaborate on projects and have ongoing collaborations with other researchers at many other institutions. I am Rebecca Whelan, an Associate Professor of Chemistry and the PI of the Whelan lab.
EKH: I’m Eliza, a fifth-year graduate student in Rebecca’s lab. I transferred to KU last year along with the lab to finish my Ph.D. degree.

Dr. Rebecca Whelan and Eliza Hanson with their lab team.
“What are your research focus areas and current projects?”
RJW: Our lab is interested in making progress on the survival statistics associated with ovarian cancer. Developing tools to enable early detection of ovarian cancer is a major goal. Much of that work is related to improving our understanding of the gold-standard serum biomarker CA125. This work takes on diverse forms, including binding affinity studies focused on the antibodies used in the clinical tests and proposing a new structural model of the protein (as we do in a recent publication in Cancer Research Communications, DOI: 10.1158/2767-9764.c.7051741) We are also interested in analytical method development in the areas of microscale separations, proteomics, and affinity reagent generation.
“What is your why? What inspires you and your team to take on some of science’s most challenging questions?”
RJW: I see myself as an educator who is also a researcher. Getting to work closely with the next generation of scientists motivates my work in both the classroom and the lab. The best part of my job as a professor is watching (and hopefully helping!) my students grow in their skills and confidence. A close second-best thing is learning from them, which I am constantly doing, since students always bring fresh perspectives and ideas. I have also been inspired by the ovarian cancer patients, survivors, and advocates whom I have been honoured to meet and converse with about our work. It is so important that scientists engaged in human health research center the voices and experiences of those affected by the disease or condition we study.
EKH: Survival rates for cases of ovarian cancer are dramatically higher when the individual is correctly diagnosed at an early stage. Unfortunately, ovarian cancer is a “silent killer” and most patients aren’t diagnosed until later stages, when the cancer has metastasized. If we could improve early detection, it would improve survival rates. I think that ultimately, there are a lot of “whys” – the really big ones, like improving survival rates, and then there are the more personal ones. I love studying chemistry, and the challenge of solving questions that haven’t been answered before makes the work more satisfying. It helps when both the end goal and the process itself are both “whys” that motivate the research.
Graduate Student, Eliza Hanson working with OpenSPR-XT in the lab.
“How long have you been familiar with our OpenSPR platform?”
EKH: We purchased an OpenSPR instrument in the early spring of 2020, and it arrived in March, just as COVID-19 forced shutdowns of many labs, including ours. As a result, we didn’t get to unbox our OpenSPR instrument until June 2020. One of my first projects, when labs reopened, was optimizing a model system on the instrument, and I’ve been using the instrument for various projects ever since.
“What inspired you to conduct a literature survey on publications leveraging OpenSPR? And what was your biggest takeaway from the study?”
EKH: In the process of writing a separate journal article about some of our work on the OpenSPR using the NTA chips (DOI: 10.3390/s23156703), I got curious about which types of surface immobilizations were most common amongst other users. In the process of trying to answer that question, I ended up doing a little bit more digging than I had planned. At that point, I started thinking about how useful it would have been to me to have a complete list of OpenSPR publications when I first started. I organized the results of my literature search and realized I had the makings of a review article. It may sound simple, but I think the thing I took away the most from this process was just how many different kinds of things people were using the SPR to do. I knew a lot of people used the instrument to measure binding kinetics and affinity (which makes sense – that’s primarily what we use it for too!) but it was fascinating to see what other applications people come up with. It made me wonder whether there were ways we could expand on our applications of the OpenSPR to answer new sorts of questions.
“Why opt to use OpenSPR instead of similar technologies or other SPR platforms on the market?”
RJW: The OpenSPR provides high-quality data at an affordable price point. It is definitely more affordable than many other SPR systems, and the benchtop instrument fits nicely into our lab, enabling us to do the experiments we design on our own schedule. Related to that, the availability of an autosampler was a huge advantage and figured strongly into our decision to buy from Nicoya. Finally, the technical and scientific support provided by members of the Nicoya team has been exemplary. When we have needed to troubleshoot, we have always felt supported by Nicoya’s customer success scientists, who are knowledgeable and helpful.
“As an experienced user, what would you consider as suitable next steps or future directions for expanding the capabilities of OpenSPR?”
RJW: In the future, we would love to see the instrument explore new capabilities such as being able to analyze samples in unprocessed biofluids. Can SPR become a routine tool in the clinic, or at the bedside? These are challenging analytical tasks, but one of the most fun things about being a bioanalytical chemist is pushing your measurement tools to their limits, asking them to perform new functions, and seeing new insights on biology that become possible as tools evolve.
EKH: In our own work, we are continuing to test affinity agents for ovarian cancer biomarker proteins, including monoclonal and polyclonal antibodies and novel nucleic acid affinity reagents.
Note: Eliza Hanson earned her Ph.D. in May 2024 and is currently working at Rowan University as a post-doctoral researcher.
5. Conclusion
In the past decade, OpenSPR has transformed biomolecular research, revolutionizing fields such as drug discovery and disease research. As highlighted in the publication, OpenSPR provides versatility and high-quality data at an affordable cost. OpenSPR’s user-friendly design, coupled with its wide range of applications, makes it the ideal instrument for every researcher’s benchtop.
As we continue to push the boundaries of SPR technology, OpenSPR remains at the forefront of innovation, empowering scientists around the world to make life-saving breakthroughs.
Discover how the versatility of OpenSPR can help you reach your research goals!
References
- Hanson EK, Whelan RJ. Application of the Nicoya OpenSpr to studies of biomolecular binding: A review of the literature from 2016 to 2022. Sensors. 2023;23(10):4831. doi:10.3390/s23104831