Digital surface plasmon resonance (SPR) offers a more automated and versatile method for studying binding properties that determine lead quality and drug efficacy.
Biomolecular interactions are at the heart of drug discovery as they promote the regulation and execution of biological processes in the body, and their characterization is critical to validating new targets and how they bind to potential drugs.
Surface plasmon resonance (SPR) is a powerful technique for observing biomolecular interactions due to its ability to quantitatively measure real-time interactions without the need for labels or markers. While SPR is a critical tool in the discovery and development of biologics, conventional systems have often been regarded as too technically challenging or resource-consuming to adopt in the early stages of the drug discovery workflow. Alto™ Digital SPR provides an alternative solution by building on the core strengths of SPR systems, while addressing common challenges and streamlining the experimental workflow. In this blog, we discuss the applications of Alto in early drug discovery.
Table of contents
- Drug discovery at a glance
- Alto: Digital surface plasmon resonance
- Applications of Alto in early drug discovery
- Conclusion
Drug discovery at a glance
The process of bringing a new drug to the market can be broken down into three stages: discovery, development and launch. Discovery includes identifying and validating a novel target, finding molecules with activity against said target, and narrowing them down to find promising leads for further optimization. Development includes a series of pre-clinical and clinical trials designed to test safety and efficacy. The whole process is time- and resource-intensive, requiring 10-15 years and over 1 billion dollars.1,2
Despite extensive research in the discovery phase, almost 90% of drugs entering clinical Phase I do not reach commercialization, indicating inefficiencies in the drug development process that result in immense resource and capital loss.3 One major reason for this outcome is poor pharmacokinetic and bioavailability profiles.4 Therefore, thorough drug-likeness profiling early in the drug discovery process can significantly improve lead quality.
Characterization of biomolecular interactions is critical throughout all stages of early drug discovery beyond just bioavailability and pharmacokinetic assessment. SPR platforms have become a standard tool in drug discovery, owing to their highly sensitive and label-free characterization of a wide range of interaction types. They provide a more robust alternative to other methods of biomolecular characterization, such as BLI and ELISA, and are frequently used at various stages of early drug discovery for detailed affinity and kinetics determination. SPR can also be used to assess pharmacokinetic (PK) parameters, bioavailability and ADME (absorption, distribution, metabolism, excretion) profiles, making it a powerful tool for early drug discovery.5
Conventional systems have often been regarded as too technically challenging or resource-consuming to adopt in the early stages of the drug discovery workflow, driving up the cost and time of therapeutic innovation. With this in mind, we developed Alto, a digitally-powered solution for automated, user-friendly SPR analysis.
Alto: Digital Surface Plasmon Resonance
Alto is the world’s first SPR instrument to integrate digital microfluidics (DMF), an advanced fluid-handling technology that precisely manipulates nanolitre sized droplets, with nanotechnology-based biosensors. Designed to accelerate drug discovery, Alto streamlines the research and development workflow to provide high-quality affinity, kinetics, quantitation, and epitope characterization data, while reducing the cost and time of discovery. With these capabilities, Alto is a valuable part of any researchers’ toolkit, providing considerable benefits for applications in early drug discovery.
Applications of Alto in early drug discovery
Target identification and validation
The first step in early drug development is the identification and validation of targets that can be leveraged to elicit a therapeutic effect. To this end, a correlation between a disease state and given genes, proteins or other biomolecules must be established. Once a target is identified, it is subjected to validation experiments to corroborate its implication in disease state and investigate whether its modulation produces a curative or therapeutic effect.
Initial target identification can be accomplished using a bioinformatics approach to mine data to identify, select, and rank candidate targets. A literature study on various genetic polymorphisms and associated diseases can also provide valuable information. Once targets have been shortlisted, they are validated using a combination of various in vitro and in vivo assays, including expression profile assays, cell-based mechanistic studies, and functional screening using knockout and knockdown models.
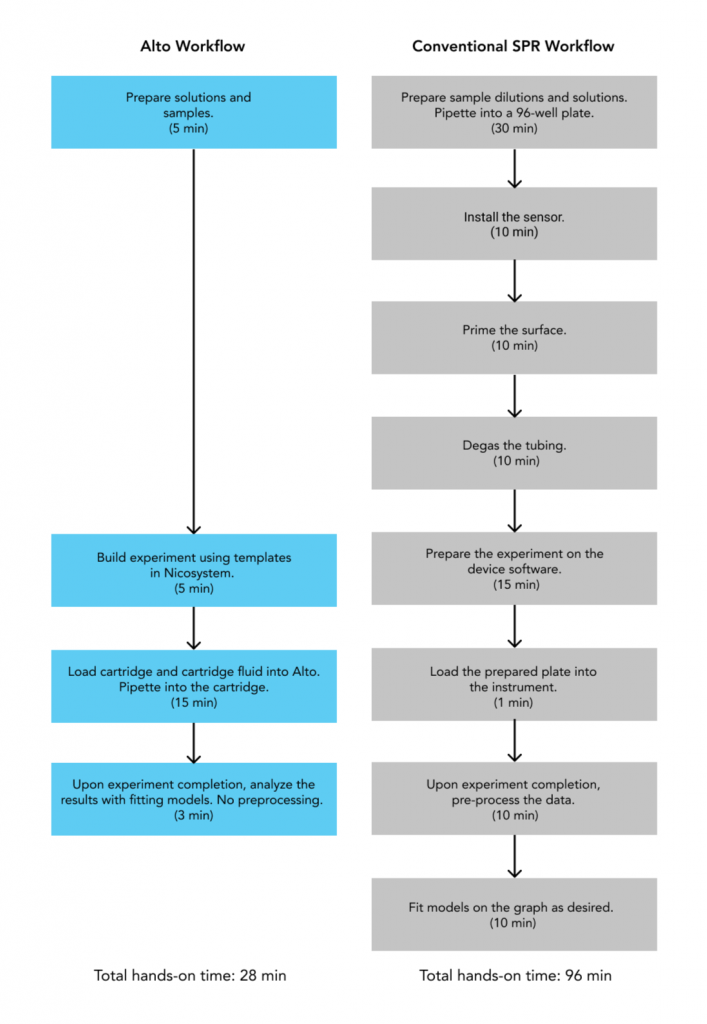
As targets need to be druggable, or have the ability to be therapeutically modulated by other molecules, characterizing their binding properties is critical in target identification and validation. SPR is an indispensable tool in this stage, as it offers a highly sensitive and label-free approach that provides real-time kinetics data, including rates of complex formation (ka), complex stability (kd) and binding affinities (KD). Alto takes this a step further by simultaneously screening up to 48 analytes, while drastically reducing sample consumption and experimental hands-on time (Figure 2).
Hit discovery and confirmation
The hit discovery phase is next, where molecules with activity against the desired target are identified and confirmed to be further validated as potential leads. This phase includes the development of compound screening assays to confirm hits and develop a hit series.
The initial discovery phase may start with knowledge-based screening, where a smaller subset of molecules with potential activity against the target molecule are selected based on the literature. Experimental approaches are also taken to identify desired molecules and further characterize them, such as ligand fishing, high throughput screening (HTS), fragment screening and ADME/PK studies. To refine candidates, molecules are evaluated to find the ones with a potency of 100 nM-5 µM at the drug target. This can be done by generating dose-response curves, in vitro assays generating ADME/PK data, amongst other methods.
Assay development is an integral part of hit confirmation to reliably assess potential hits, define a hit series, and select the most promising hits, which are known as leads. While the choice of assay depends on experimental goals, assays should be selected for pharmacological relevance, and developed to accurately characterize molecular properties, be reproducible across plates and users, and be robust.
SPR is a gold standard tool for streamlining the hit discovery and confirmation phase, including assay development, as it provides a molecule-agnostic, reproducible approach for binding affinity and kinetics analysis. The detailed kinetics data enable early ADME/PK and bioavailability analysis. SPR can also be used to generate dose-response curves and to conduct structure-activity (SAR) relationship studies.
Alto further enhances hit confirmation studies by automating all sample manipulation and data analysis to standardize assays and eliminate user-to-user variability. Additionally, Alto’s fluidics-free approach unlocks crude sample compatibility, which is often a limitation of fluidics-based SPR systems. This allows for screening of compounds in complex media, thus broadening its application in fishing experiments and determination of compound interaction with serum proteins, which has important implications for early ADME/PK and bioavailability assessment.
Hit to lead and lead optimization
Hits are confirmed and validated to narrow down candidates with the highest potential, known as leads, which are then subject to further validation before entering the pre-clinical phase of drug development. Accurate characterization in this phase is important for failing low quality candidates as early as possible and maximizing downstream efficiency. SPR biosensors are especially suited for this phase of drug development for several reasons.
Traditional end-point assays only provide limited affinity and concentration data, and can only assess one interaction characteristic per assay. Instead, SPR is able to generate comprehensive data on multiple interaction characteristics in parallel, including affinity, kinetics, concentration, and specificity, allowing the user to eliminate low quality candidates in one fell swoop and minimize the hands-on time required for experimentation.
Additionally, SPR analysis can complement pharmacodynamics studies by providing more information on key drug properties, including ADME characteristics, bioavailability, potency, and thermodynamics. For instance, the extent of binding to plasma proteins is an important determinant of bioavailability and ADME profiles, and high-affinity to serum proteins is a common source of failure for drug candidates in clinical trials. SPR can be used to discard these molecules early on to improve the success rate of candidates entering clinical trials. Furthermore, kinetics information provided by SPR can be used to calculate the affinity (KD) and change in free energy (DG) of complex formation, which are important parameters in determining therapeutic potential. For these reasons, the comprehensive data generated by SPR platforms has been shown to significantly reduce the number of hits brought to the lead stage, preventing unnecessary resource and time investment into low quality leads.6
Alto further introduces efficiencies by minimizing sample requirements and hands-on time, freeing up precious resources and time that can be reinvested elsewhere. By automating several aspects of the workflow, including assay design and sample preparation, Alto empowers users with a plug-and-play system that standardizes experimental protocols and eliminates operator variation. To demonstrate its effectiveness in assessing candidates for clinical potential, we’ve characterized a wide range of drug modalities including bispecifics, interleukin receptors and antibody drugs, biosimilars, and oligonucleotides.
Conclusion
Lead selection and optimization are bottlenecks in the drug discovery pipeline and important targets for process streamlining. In addition, the eventual failure of almost 90% of drug candidates entering Phase I clinical trials signals a pressing need for better lead optimization. Faster and more robust characterization methods are needed to assess drug-likeness and fail low quality leads earlier in the process. SPR can play a vital role in simplifying early drug development given its speed, accuracy and data robustness, but is limited by accessibility and high operating costs.
Our next-generation SPR platform, Alto, builds on the capabilities of conventional SPR systems by providing an automated platform that streamlines research workflow, eliminates user-to-user variation, and significantly minimizes the sample and time requirements. For researchers looking to refine their drug discovery workflow, zero in on high quality leads, and enhance their pipelines without compromising on data quality, Alto is the answer.
Streamline your early drug discovery with Alto. Chat with us today to learn how!
References
- Turner JR, Hoofwijk TJ. Clinical trials in new drug development. J Clin Hypertens (Greenwich). 2013;15(5):306-309. doi: 10.1111/jch.12085
- Wouters OJ, McKee M, Luyten J. Estimated research and development investment needed to bring a new medicine to market, 2009-2018. JAMA. 2020;323(9):844-853. Doi: 10.1001/jama.2020.1166
- Hay M, Thomas DW, Craighead JL, Economides C, Rosenthal J. Clinical development success rates for investigational drugs. Nat Biotechnol. 2014;32(1):40-51. doi: 10.1038/nbt.2786
- Waring MJ, Arrowsmith J, Leach AR, et al. An analysis of the attrition of drug candidates from four major pharmaceutical companies. Nat Rev Drug Discov. 2015;14(7):475-486. doi: 10.1038/nrd4609
- Löfås S. Optimizing the hit-to-lead process using SPR analysis. Assay Drug Dev Technol. 2004;2(4):407-415. doi: 10.1089/adt.2004.2.407
- Fabini E, Danielson, UH. Monitoring drug–serum protein interactions for early ADME prediction through Surface Plasmon Resonance technology. J Pharm Biomed Anal. 2017;144:188-194. doi: 10.1016/j.jpba.2017.03.054

