Alto™ streamlines the characterization of Fc gamma receptors in our latest app note comparing analysis with Digital SPR and BLI.
Fc receptors (FcR), named for their binding specificity to the fragment crystallizable region of antibodies, play a key role in myriad biologic pathways. As FcR dysregulation is implicated in a wide range of autoimmune disorders, cancers, and infectious diseases, analytical tools able to effectively elucidate their molecular mechanisms are imperative in developing FcR-targeted therapeutics. However, the demanding workflows of current analytical instruments often do not meet the needs of the modern biologics researcher, given their complex and resource-consuming protocols.
We developed Alto, our Digital SPR platform, to streamline biomolecular characterization and eliminate the challenges of conventional fluidics-based analysis. In a recent application note, we demonstrated Alto’s applicability in FcR-targeted therapeutics development with a case study on Fc gamma receptor I (FcγRI) affinity and kinetics analysis, while providing a side-by-side comparison to biolayer interferometry (BLI) analysis. Continue reading below to see how Alto’s ultra-low sample and hands-on requirements accelerate therapeutics development, or read the full application note.
Table of contents
- Fc receptor-targeted therapeutics
- Comparison of label-free analytical methods for FcγR applications
- Characterizing antibody binding to FcγRs with Digital SPR
- Digital SPR vs BLI
- The verdict
Fc receptor-targeted therapeutics
Named for their binding specificity to the Fc (fragment crystallizable) region of antibodies, FcRs are known to target infected cells or invading pathogens, and recruit phagocytic or cytotoxic cells to destroy their targets via antibody-mediated phagocytosis or antibody-dependent cell-mediated cytotoxicity.1, 2 FcRs are classified according to the type of antibody they recognize, with one example being the Fc gamma receptor (FcγR) that binds to immunoglobulin G (IgG).2 As FcγR dysregulation is implicated in a wide range of diseases, elucidation of its function is critical to developing therapeutics that target and treat these diseases.
Comparison of label-free analytical methods for FcγR application
Label-free analytical methods are used in target molecule identification to lead selection and optimization during the therapeutic discovery workflow. Several label-free approaches can be used, including biolayer-interferometry (BLI) and conventional surface plasmon resonance (SPR). While conventional SPR has higher sensitivity and provides more robust data compared to BLI, many researchers opt for BLI given its crude sample compatibility, higher throughput, cost savings, and fluidics-free design. Our digital SPR technology offers a better alternative by providing the advantages of both conventional SPR and BLI platforms in the form of a low-footprint, user-friendly device.
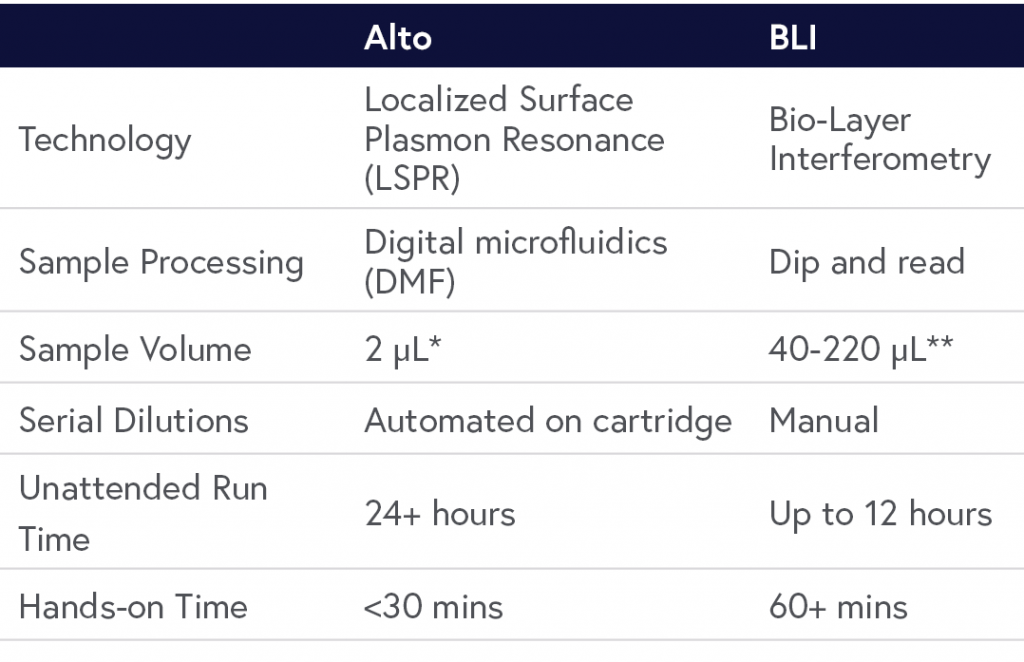
BLI platforms use an interference pattern of white reflected light from immobilized protein on the sensor tip to collect data using a dip and read method. While this approach eliminates fluidics hardware, it requires a minimum of 40-220 µL of sample per concentration. It is also limited in measurement time due to evaporation and often exhibits significant mass transport effects, limiting the experimental affinity and kinetic range.
In contrast to BLI, Alto employs localized SPR (LSPR) technology that is powered by digital microfluidics (DMF). All reagents are loaded into a self-contained disposable cartridge and manipulated using voltage changes. When the experiment is complete, the cartridge is easily discarded and the data are readily analyzed with the click of a button. Altogether, Alto requires minimal training to use, is compatible with crude samples, and requires only 2 µL sample volumes for full kinetic characterization across a wide range of affinity and kinetics. A comparison of Alto to BLI is summarized in Table 1.
Table 1: Comparison of Alto Digital SPR and BLI.
Characterizing antibody binding to FcγRs with Digital SPR
Alto presents several advantages for characterizing FcγRs and their targets, compared to other label-free tools such as BLI. Its 16 independent channels allow the simultaneous analysis of multiple targets in a variety of assay formats, while nanoscale sample manipulation and automated dilutions introduce a high-degree of precision in an otherwise error-prone technique. In addition, the ability to control the flow rate, as compared to the dip and read method of BLI, enables researchers to better optimize assays and mitigate potential effects of mass transport. These advantages allow for rapid and in-depth characterization of FcγR-IgG interactions to assess binding affinity and efficacy of novel antibody-based therapeutics.
To demonstrate Alto’s applicability in FcγR characterization, we developed an assay to measure binding between mouse Fc gamma receptor I (FcγRI) and the mouse antibody IgG2A through several experimental approaches. A similar analysis was also performed using a standard BLI instrument.
Digital SPR vs BLI
Workflow
A side by side experimental workflow comparison revealed Alto required 28 min of hands-on operator time for full analysis, compared to 66 min using the BLI. Alto’s automated on-cartridge serial dilutions reduced sample preparation to a sixth of the time, while data analysis with the Nicosystem software reduced analysis time to a third of the time required with BLI.
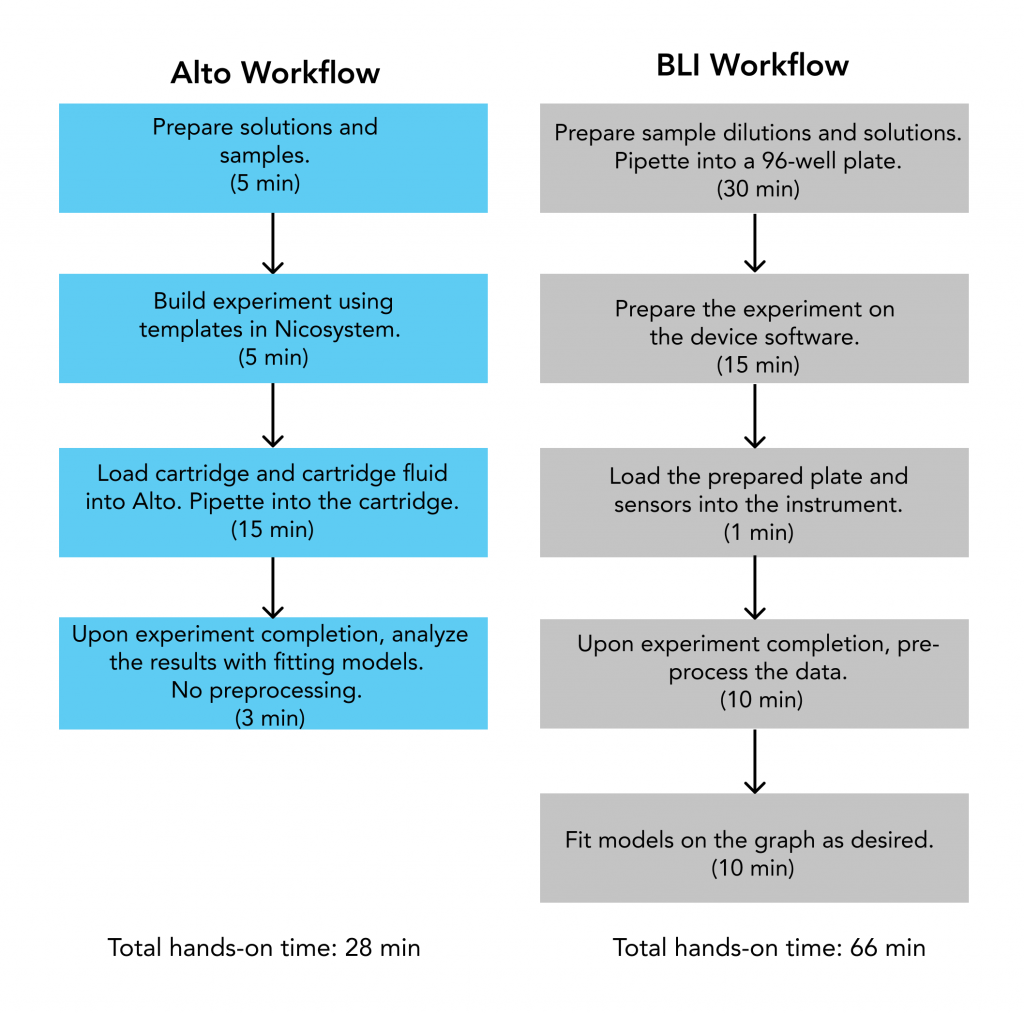
Results
While Alto demonstrated equivalent accuracy for obtaining affinity and kinetics measurements as BLI, all aspects of the experiment were automated by Alto to reduce the total experimental time by over 50%. Leveraging DMF technology, Alto also performed all sample dilutions and analyses on a single cartridge using just a fraction of the sample needed for analysis with BLI. The results highlighted Alto’s ability to provide high-quality data while reducing time to answer, proving its viability for streamlining FcR and antibody research.
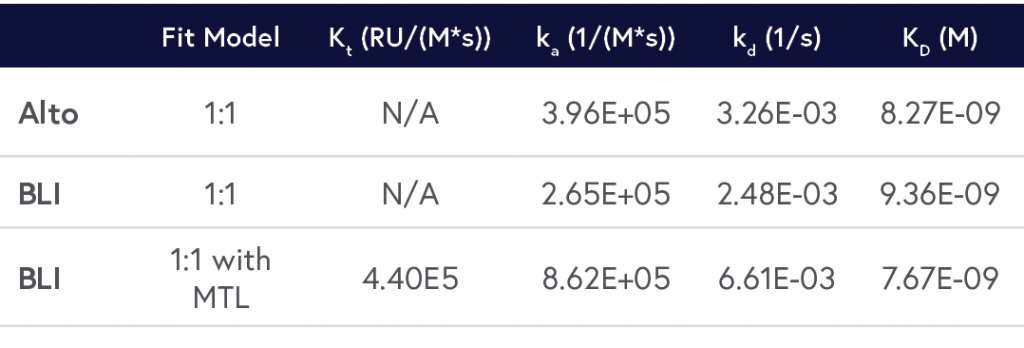
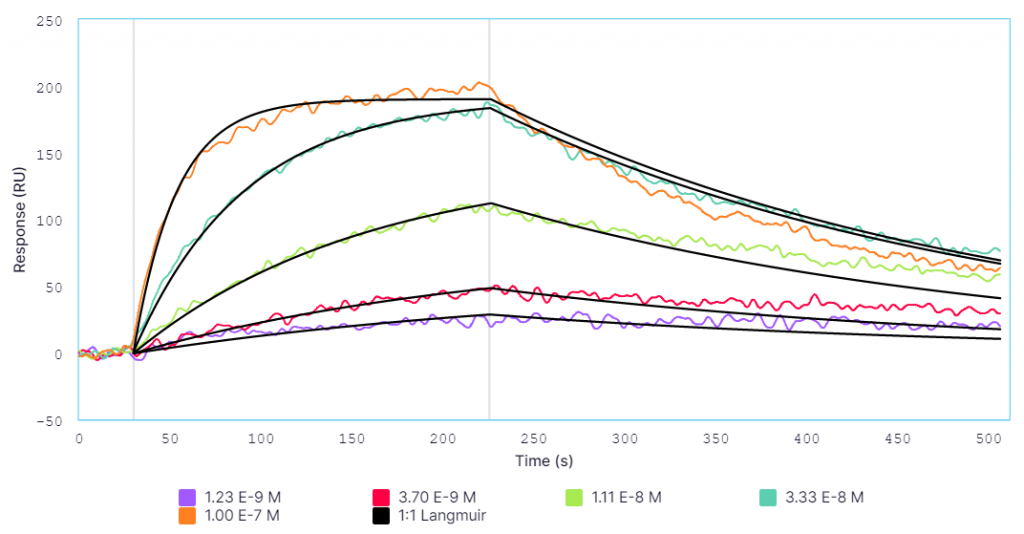
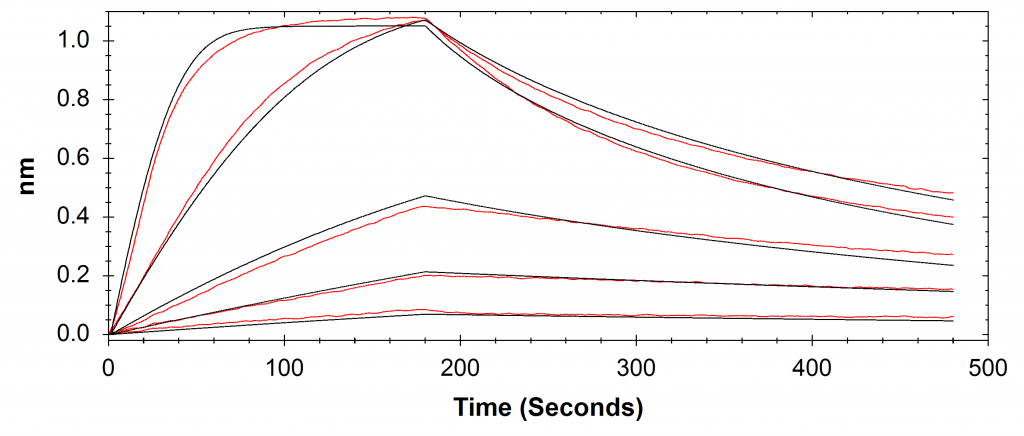
A comparison of results between Alto and BLI has been provided in Table 2 and respective sensorgrams are shown in Figures 2 and 3.
Table 2: Comparison of the kinetic data parameters calculated from the sensorgrams collected by Alto and BLI experiments.
The verdict
With FcγR being implicated in a wide range of autoimmune diseases and cancers, analytical tools able to effectively elucidate their molecular interactions are imperative for devising targeted treatments.
By crunching the evidence above, it’s easy to see why Alto is the superior approach for characterizing biomolecular interactions. Alto generated data comparable to standard BLI platforms without requiring mass transport limitation (MTL) correction, while using just over 40% of the hands-on time and 0.2% of the sample volume.
Alto is the optimal fit for the modern biologics developers striving to develop therapeutics without compromising on efficiency. To learn more about how Alto can accelerate your biologics development pipeline, check out the full application note, or get in touch with our team for an in-person demonstration!
Read the full application note today.
Read Application NoteReferences
1. Takai T. Roles of Fc receptors in autoimmunity. Nat Rev Immunol. 2002 Aug;2(8):580-92. doi: 10.1038/nri856. PMID: 12154377.
2. Junker F, Gordon J, Qureshi O. Fc Gamma Receptors and Their Role in Antigen Uptake, Presentation, and T Cell Activation. Front Immunol. 2020 Jul 3;11:1393. doi: 10.3389/fimmu.2020.01393. PMID: 32719679; PMCID: PMC7350606.