Have you ever ordered antibodies from an antibody supplier? Have you ever had a problem with antibody specificity, selectivity and reproducibility? Have you ever returned your antibodies back to the supplier only to receive another poor quality sample? If you answered yes to the questions above, you’ve suffered from the pandemic of “bad” antibodies. It’s estimated that roughly 50% of all purchased antibodies are “bad” which accounts for roughly $350 million USD in wasteful spending in the US alone. With well over 4 million Research Use Only (RUO) antibodies available to investigators, it’s important to remember two things; source well and cross validate in-house wherever possible. In this blog, we discuss how you should validate parameters (i.e. selectivity, specificity on/off rates, equilibrium constants) of purchased antibodies to ensure you’re receiving a high quality antibody that is useful for your applications, saving you time and money.
3 Types of Antibodies
In the world of antibodies, there are 3 major classes of antibodies; polyclonal, monoclonal and recombinant. Polyclonal and monoclonal antibodies are generated in the immune system by memory B-cells which secrete antibodies in response to a foreign antigen. A polyclonal antibody holds the capability to recognize and bind different epitopes on the same antigen while a monoclonal antibody recognizes and binds a single epitope on the antigen. The main difference between these antibody classes lies in their specificity; monoclonal antibodies are highly specific, while polyclonal antibodies are more likely to cross-react and thus, decrease specificity. Notably, recombinant antibodies (ie. those produced in vitro by DNA recombination, expression, purification etc.) behave in a similar fashion to monoclonal antibodies but come with a number of production advantages including rapid production, faster turnaround and no need for a host animal; it is theorized that recombinant antibody technology will become the main antibody production method (Bradbury, A., & Plückthun, 2015).
Issues with Antibody Quality
Over the last few decades, there has been a shift from traditional small molecule drugs to antibody-based therapies and in this respect, the demand for commercially available antibodies has spiked. This has resulted in an explosion of new producers and distributors paving the way for an estimated market value of over $22 billion USD by 2025 (Grand View Research, 2017). It should be noted however, with any upward market trend, the issue of standardization and quality assurance typically follows.
Batch-to-batch variability is a flaw that is inherent to the traditional production methods of antibodies. Hybridomas, the vector in which monoclonal antibodies are produced, are subject to genetic drift which basically means that the antibody you’ve based an entire project around for years, may not possess the same selectivity, specificity and/or capacity as was previously expected, leading to severe project headaches, as well as wasted time and money.
Although many antibody producers and antibody distributors provide a Certificate of Analysis (CoA), Western blots and/or ELISA data, there are some companies that do not provide this information, so it is important to maintain vigilance, be a smart consumer and to source antibodies wisely. Furthermore, many producers that have validated antibodies with techniques such as Western blots or ELISA will claim that the antibodies are suitable for a wide variety of applications (ie. IHC or flow cytometry), with no supporting documentation. With that said, there has been an emergence of in-house antibody quality assurance. Validating parameters such as selectivity, specificity, sequence data and on/off rates and equilibrium constants of purchased antibodies can hold producers accountable and ensure you’re receiving a high quality antibody that is useful for your applications.
Binding Constants and Surface Plasmon Resonance
In a 2016 paper published in the journal, Analytical Chemistry Insights, the authors state that since antibody binding constants are static properties and only small fractions of antibodies are sold with affinity data, “it is a pity that the determination of affinity constants is rarely performed for antibodies and their respective antigens” (Weller, 2016). In the same publication, the authors posture that the most welcomed approach to characterizing binding constants and validate antibody quality is surface plasmon resonance (SPR). There are other technologies available to characterize binding constants but unfortunately these techniques typically use a lot of sample, require labels, do not provide enough information and/or require large capital expenditure. Notably, surface plasmon resonance (SPR) has been the gold standard for obtaining quantitative binding kinetics data for a number of decades.
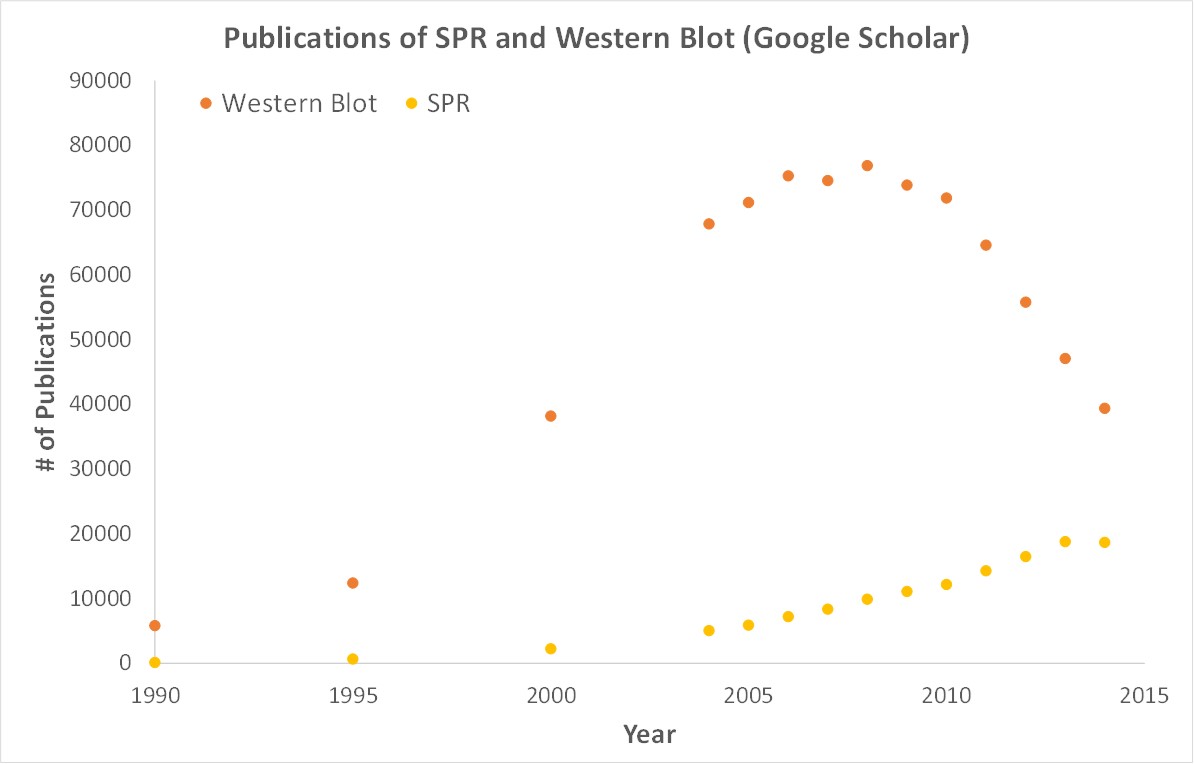
Importantly, OpenSPR is a user-friendly and low maintenance benchtop SPR solution that is currently being used by hundreds of researchers. With access to SPR technology on your own lab bench, you can get the high-quality data you need to publish successfully. SPR is necessary not only for publications, but for the advancement of many fields of medicine and medical research as can be seen below with the significant increase in publications that rely on SPR data.
Resources
- Antibody Production Market Worth $22.6 Billion By 2025. (2017, March). Retrieved June 10, 2019, from https://www.grandviewresearch.com/press-release/global-antibody-production-market
- Bradbury, A., & Plückthun, A. (2015). Reproducibility: Standardize antibodies used in research. Nature, 518(7537), 27-29. doi:10.1038/518027a
- Weller, M. G. (2016). Quality Issues of Research Antibodies. Analytical Chemistry Insights, 11. doi:10.4137/aci.s31614
