Overview
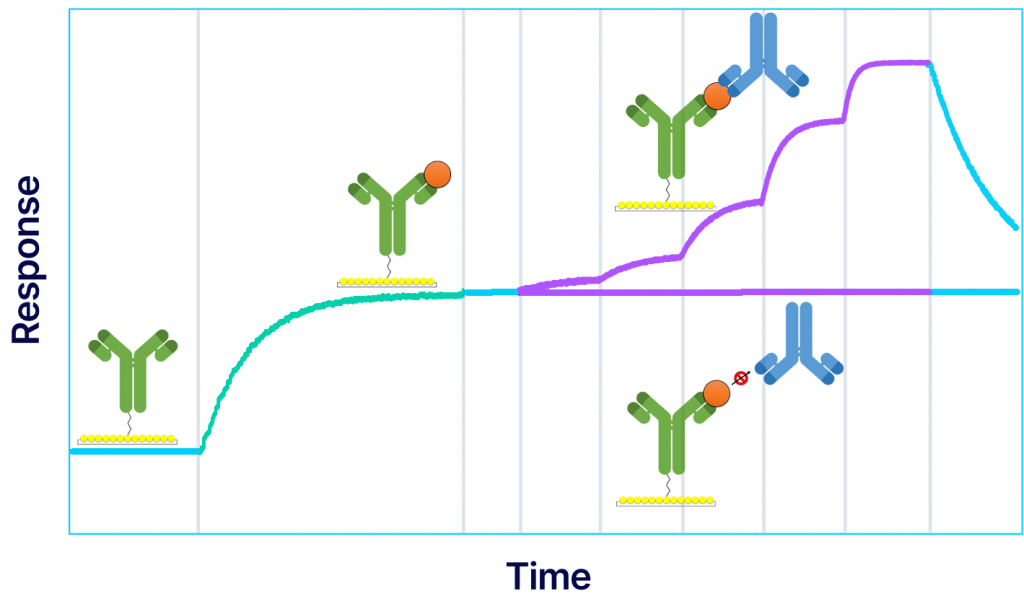
Annual formulation updates for influenza vaccines and related antibody therapies are required to preserve immune recognition against different influenza subtypes. Characterizing the binding kinetics and epitope diversity of various antibodies to influenza viral antigens is essential for treating and preventing potential outbreaks. In collaboration with Sino Biological, a global leader in recombinant technology, we use our Alto system to perform kinetic analysis and epitope characterization of several antibodies against influenza A hemagglutinin (HA), using DMF-powered surface plasmon resonance (SPR) technology. Alto provides a streamlined automated assay to achieve accurate kinetics data with 200x less sample consumption.