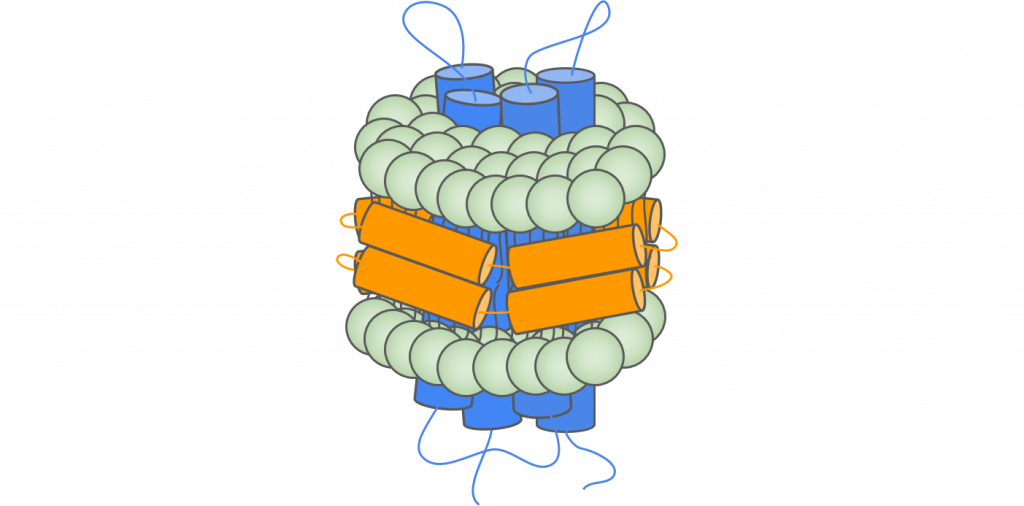
Overview
Many human diseases are known to be associated with transmembrane proteins (TPs), making them ideal candidates for drug development. Multi-pass transmembrane proteins are not stable outside of the cell membrane environment, and are therefore difficult to purify and to express in large quantities. This has resulted in the under-characterization of a critical group of biomolecules. One strategy for overcoming these challenges is to use vectors that closely mimic cell membranes, such as nanodiscs. In this application note, Alto Digital SPR was used to characterize binding kinetics of Claudin-18.2 in nanodiscs from ACROBiosystems, demonstrating its ability to provide high-quality data while significantly reducing precious sample consumption and time to results.