Binding analysis with surface plasmon resonance (SPR) provides both affinity and kinetics data, giving you the bigger picture.
As one of the most standardized and well-characterized immunoassays performed in the lab, the ELISA (enzyme-linked immunosorbent assay) is repeatedly recognized as the gold standard for detecting antibodies, proteins, and other biomolecules for a variety of research and clinical applications. Owing to its high sensitivity, specificity, and accessibility, the ELISA is often used as the standard comparison for evaluating new assay technology, and is the method of choice for those looking for a cost-effective approach for obtaining quantitative interaction data. However, the labour-intensive nature of the assay compounded with the increasing complexity of scientific questions necessitates the use of analytical tools that can unlock real-time data, while minimizing barriers for the user.
The past few decades have seen the emergence of surface plasmon resonance (SPR) as a preferred method for biomolecular detection over more traditional techniques such as the ELISA. As a label-free, real-time assay, SPR overcomes many of the limitations of ELISAs while providing greater sensitivity and specificity for challenging applications. Below, we conduct a side-by-side comparison of ELISA and SPR to review the overall usability of each method.
Table of contents
- Enzyme-linked immunosorbent assay (ELISA) overview
- Surface plasmon resonance (SPR) overview
- ELISA vs SPR: A side-by-side comparison
- Nicoya’s benchtop SPR solutions
Enzyme-linked immunosorbent assay (ELISA) overview
Enzyme-linked immunosorbent assays (ELISAs) are standard practice in most academic and commercial laboratories for detection and quantification of antibodies, proteins, peptides, and other biomolecules of interest. As a plate-based assay, the overarching premise of an ELISA involves immobilizing a target biomolecule (antigen) to a solid surface, and complexing it with an antigen-specific antibody that is linked to a label or tag for detection. Binding is detected via a secondary reaction that is produced by the label and measured with microplate readers.
While ELISA is often the method of choice for quantifying biomolecules and detecting antibody-antigen interactions, there is an increasing shift away from this conventional assay for certain applications where more powerful, automated tools are required. An alternative method to consider is surface plasmon resonance.
Surface plasmon resonance (SPR) overview
SPR is an optical detection method that leverages changes in the refractive index for characterizing biomolecular interactions. The basis for SPR assays involves immobilization of one binding partner, the ligand, on the sensor surface, followed by the injection of the second binding partner, the analyte, over the sensor surface (refer to Figure 1). When binding occurs, this produces a real-time signal that can be used to measure binding affinity and kinetics.
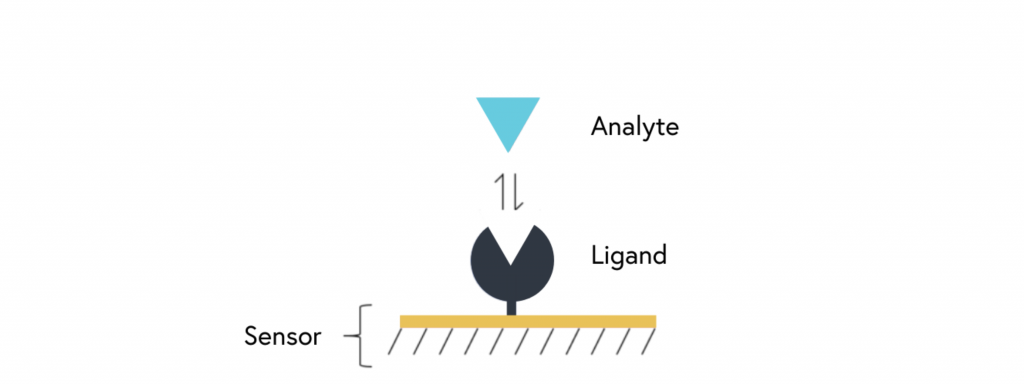
SPR is one of few techniques that allow for accurate and real-time determination of binding kinetics, providing researchers with more detailed information about the molecules and systems being studied. As a result, it lends itself well to applications such as ligand-receptor and enzyme-substrate interaction studies, drug screening, epitope mapping, protein conformation studies, and label-free immunoassays.
ELISA vs SPR: A side-by-side comparison
Data measurement
An ELISA is an end-point assay that allows the user to detect the presence of a target biomolecule, often from a complex mixture. In doing so, one is able to measure and quantify high-affinity proteins and antibodies in biological samples. However, ELISA data only give insight into the amount of biomolecules of interest present within a given sample, and as a result do not provide information on the kinetics between the target and the bound biomolecule. For applications in drug discovery and disease mechanism research, it is especially critical to measure both binding affinity and kinetics to elucidate the true binding of a biomolecule with its target. To put things in perspective, interactions with the same binding affinity can have binding constants varying by 4 orders of magnitude.
In contrast, SPR provides more in-depth characterization of specific interactions due to its ability to observe binding in real time. Using a small amount of sample, SPR is able to determine both binding affinity and kinetics, providing valuable information on the association and dissociation phases of the interaction of interest. Consequently, researchers can use this data to determine which molecules interact, why they interact, and how strongly they interact.
Our winner: SPR
Label requirement
An ELISA depends on the use of tagged antibodies and substrates to generate a measurable signal. Depending on the format of the assay, multiple antibodies can be used to finetune the sensitivity and specificity of the assay design. The drawback is that they have to be carefully selected and optimized to avoid cross-reactivity, which adds to the complexity and time requirements of ELISAs.
SPR provides a direct and label-free alternative, as binding is measured via a change in refractive index. This streamlines the assay design, optimization, and execution.
Our winner: SPR
Experiment length
The ELISA format permits a high degree of customizability, but increases the assay time requirements. Running an experiment includes long coating, incubation, washing, blocking, and signal generation steps, as well as significant hands-on time.
SPR protocols are simplified by the direct acquisition of binding data based on changes in the refraction index. In addition, washing and sensor preparation steps can be completed in the SPR instrument itself. All of this greatly reduces the time to answer in SPR experiments in comparison to ELISAs, which usually take longer than a day. Refer to Figure 2 for a side-by-side comparison of our digital SPR system Alto and an ELISA.

Our winner: SPR
Low-affinity interactions
ELISAs have been reported to characterize high-affinity interactions with equal or higher sensitivity than other, more complex, methods.2,3 However, they fall short when it comes to characterizing low-affinity interactions, which are more scientifically and clinically relevant in certain applications. As the ELISA signal is based on the protein-target binding affinity, low-affinity activity can result in a weak signal. Low-affinity binders are often lost during the multiple washing steps in an ELISA. This makes it difficult to determine whether the weak signal is due to low affinity or poor expression of the protein, and can lead to false-negative results.
In contrast, studies have shown SPR to effectively quantify both low-affinity and high-affinity interactions.3-5 With the ability to observe binding in real-time, SPR offers the sensitivity to measure a wider range of affinities, making it a more versatile and reliable method for detecting low-affinity events.
One example of this is quantifying low-affinity anti-drug antibodies (ADA) generated in response to therapeutic monoclonal antibodies (mAbs).4 As patient response to mAbs can range from having no clinical implications to developing severe life-threatening conditions, it is imperative to quantify these low-affinity interactions during all stages of drug development to better understand the impact of ADAs.6,7 In a study by Lofgren on the detection of low-affinity ADAs, SPR detection helped to identify a positivity rate of 4%, compared to 0.3% by ELISA.2 As well, SPR consistently showed higher sensitivity in detecting low-affinity interactions, thus suggesting SPR to be the better technique for this clinical application.
Our winner: SPR
Operating and maintenance costs
The ELISA is a highly cost-effective and accessible analytical tool, which has led to its widespread use over the years. Most laboratories are already equipped with pipettes, microplates, and microplate readers, all of which can be used for a multitude of assays and only require standard maintenance at regular intervals. While commercial kits are available to streamline ELISAs, researchers can opt to develop and optimize assays in-house to further minimize costs.
While Nicoya’s robust and low-maintenance platforms lower the ongoing costs of operating SPR, most other systems on the market tend to have high upfront costs and are typically purchased with maintenance packages, as these fluidics-based systems are prone to clogging and require frequent servicing. Instrument repair can only be done by trained technicians, which often results in down time that halts research. In contrast, our digital SPR platform Alto completely eliminates fluidics maintenance. Troubleshooting on our SPR systems can be conducted from the comfort of your own lab with the guidance of one of our Customer Success Scientists, and units can easily be shipped for maintenance.
Our winner: ELISA and Nicoya SPR systems
Learning curve
The widespread adoption of ELISAs in academic and commercial labs can be partly attributed to the short learning curve associated with the method. Basic transferable skills such as pipetting and knowledge of antibodies provide the basis for designing and conducting ELISAs.
In contrast, most SPR systems on the market require extensive training and years of technical experience to produce high quality data, which we have addressed in designing OpenSPR and Alto. Our platforms have been designed with the user in mind, shortening the learning curve by automating processes and integrating an intuitive software that provides seamless user experience from assay design to data analysis.
Our winner: ELISA and Nicoya SPR systems
The verdict
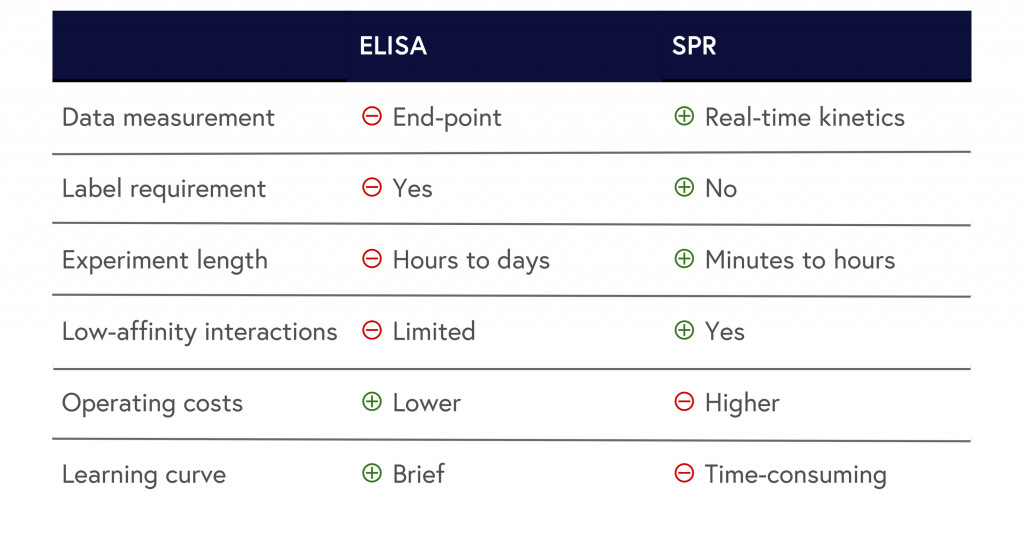
While SPR and ELISA each have their own advantages and disadvantages, our analysis concludes that SPR clearly provides an edge. We’ve summarized the results below to show how SPR outperforms ELISA in most criteria assessed in this article:
Table 1: Summary of ELISA and SPR comparison.
Our winner: SPR
Nicoya’s benchtop SPR solutions
Many researchers continue to opt for ELISAs as a seemingly more cost-effective and accessible approach, potentially forfeiting valuable data and critical workflow advantages that are accessible from SPR. At Nicoya, our benchtop SPR solutions leverage localized SPR (LSPR) and digital microfluidics (DMF) technology to provide the advantages of both conventional SPR and ELISA in the form of low-footprint, cost-effective, and user-friendly devices.
As the world’s only LSPR platform to integrate DMF technology, Alto is the perfect companion to your workflow for real-time detection and characterization of a wide range of biologics applications. With simultaneous detection across 16-channels, Alto provides high-throughput analysis of up to 48 unique targets, while further streamlining user workflows with automated sample dilutions, disposable fluidics, and sample requirements reduced by up to 200X.
Our benchtop LSPR solutions are the perfect fit for the modern biological researcher looking to reduce time to answer without compromising on data quality. Make the switch to LSPR today by chatting with one of our application scientists!
Make the switch to LSPR today by chatting with one of our application scientists.
References
1. General Sandwich ELISA Protocol. Thermo Fisher Scientific Inc. Accessed January 25, 2023. https://www.thermofisher.com/ca/en/home/references/protocols/cell-and-tissue-analysis/elisa-protocol/general-elisa-protocol.html
2. Lofgren JA, Dhandapani S, Pennucci JJ, et al. Comparing ELISA and surface plasmon resonance for assessing clinical immunogenicity of Panitumumab. J Immunol. 2007;178(11):7467-7472. doi: 10.4049/jimmunol.178.11.7467.
3. Barger TE, Kuck AJ, Chirmule N, Swanson SJ, Mytych DT. Detection of anti-ESA antibodies in human samples from PRCA and non-PRCA patients: an immunoassay platform comparison. Nephrol Dial Transplant. 2012;27(2):688-693. doi: 10.1093/ndt/gfr213.
4. Nechansky A. HAHA – nothing to laugh about. Measuring the immunogenicity (human anti-human antibody response) induced by humanized monoclonal antibodies applying ELISA and SPR technology. J Pharm Biomed Anal. 2010;51(1):252-254. doi: 10.1016/j.jpba.2009.07.013.
5. Beeg M, Burti C, Allocati E, et al. Surface plasmon resonance unveils important pitfalls of enzyme‑linked immunoassay for the detection of anti‑infliximab antibodies in patients’ sera. Sci Rep. 2021;11(1):14976. doi: 10.1038/s41598-021-94431-x.
6. Koren E, Smith HW, Shores E, et al. Recommendations on risk-based strategies for detection and characterization of antibodies against biotechnology products. J Immunol Methods. 2008;333(1-2):1-9. doi: 10.1016/j.jim.2008.01.001.
7. De Groot AS, Scott DW. Immunogenicity of protein therapeutics. Trends Immunol. 2007;28(11):482-490. doi: 10.1016/j.it.2007.07.011.
