Age is the greatest risk factor for developing cancer. According to the American Society of Clinical Oncology, 60% of people who have cancer are 65 or older [1]. Furthermore, the same mechanisms of aging are found to occur in carcinogenesis, including the role of cellular senescence [2]. Cellular senescence is the irreversible arrest of cell proliferation that occurs when cells experience oncogenic stress [2]. Since aging and cancer are tightly interconnected, many of the same strategies and drugs may be used to target both [3]. Dr. Braden Roth, a researcher at the Medical University of South Carolina, uses OpenSPR’s localized surface plasmon resonance technology to provide him with the key binding data needed for his recent publication. The research publication “Balance between senescence and apoptosis is regulated by telomere damage–induced association between p16 and caspase-3” uses binding data generated with OpenSPR to discover a link between telomere damage–dependent senescence and apoptosis with regards to aging and cancer.
About the Publication
Telomerase activation protects cells from telomere damage by delaying senescence and inducing cell immortalization, whereas telomerase inhibition mediates rapid senescence or apoptosis [2]. Telomerase activation occurs in majority of cancer cells, to induce cell immortalization and tumorigenesis [2]. However, the cellular mechanisms that determine telomere damage–dependent senescence versus apoptosis induction are largely unknown. This publication demonstrates that in lung cancer cells, the interaction between p16 and caspase-3 proteins forces senescence induction by inhibiting caspase- 3 activation and apoptosis. The binding between recombinant and purified human p16 and caspase was studied using surface plasmon resonance.
Why was OpenSPR instrumental for this research?
Surface plasmon resonance (SPR) was used to determine the binding between recombinant and purified human p16 and caspase 3 proteins at various protein concentrations. P16 was immobilized on Nicoya’s carboxyl sensor chip, and the protein-protein experiment was run on the OpenSPR system. By using OpenSPR, Dr. Roth was able to determine the kinetic constants (association constant, dissociation constant and affinity) of the interaction between p16 with caspase 3. The data showed that p16 interacted with caspase 3 with an equilibrium dissociation constant (KD) of 1.82 x 10-8 +/- 6.5 x 10-9 M, which suggests that p16 binding might be preventing the activation of pro-caspase 3 and apoptosis in response to telomere damage. In conclusion, these results show that p16 plays a direct role in telomere damage–dependent senescence by limiting apoptosis via binding to caspase-3, revealing a direct link between telomere damage–dependent senescence and apoptosis with regards to aging and cancer. With OpenSPR, Dr. Roth’s research lab was able to get SPR data from their own bench. This helped them accelerate their research and publish their discovery faster.
Why is SPR critical for publications? How does OpenSPR help?
SPR is a label-free technology which allows researchers to quantitatively analyze binding between two biomolecules. SPR technology allows us to determine the kon, koff and KD of interactions, providing deeper insight into binding events compared to other techniques that only give endpoint measurements, such as pull-down assays. SPR is necessary not only for publications but for the advancement of many fields of medicine and medical research as can be seen below with the significant increase in publications that rely on SPR data.
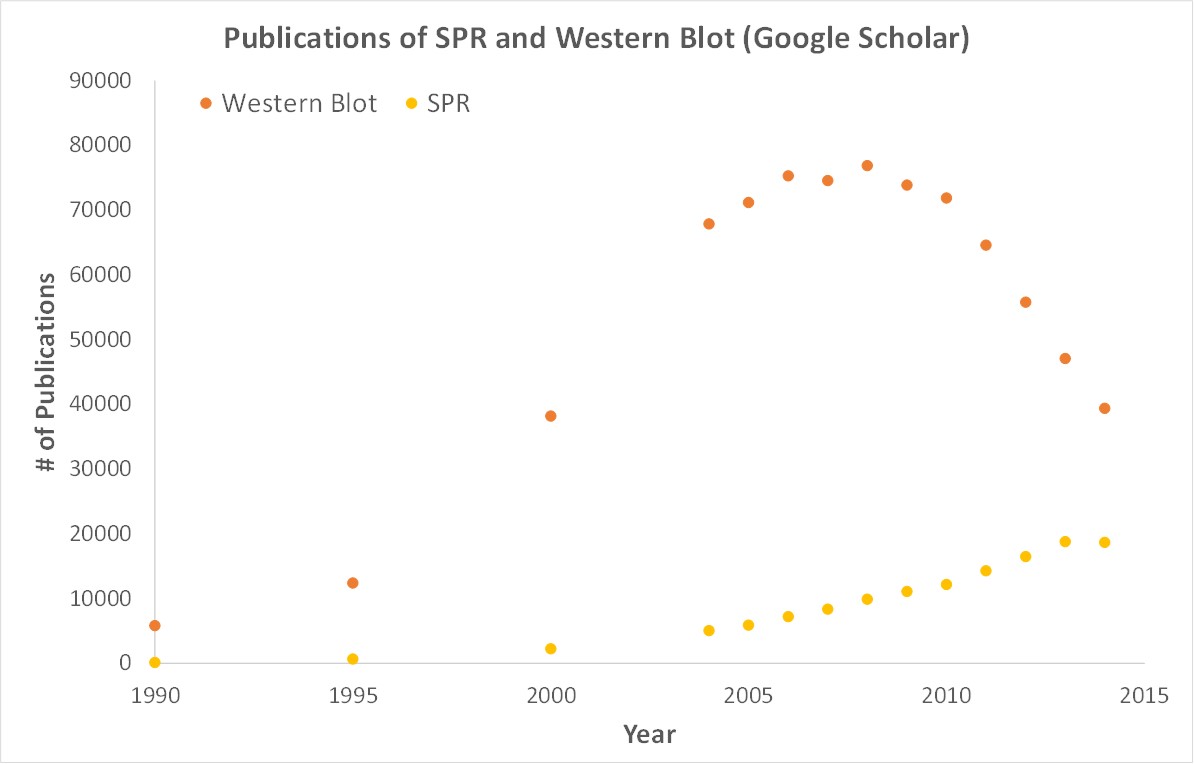
Scientific publications involving SPR have increased drastically over the years. SPR has become fundamental for publications while traditional techniques like Western Blots are becoming less important.
OpenSPR is a user-friendly and low maintenance benchtop SPR solution that is currently being used by hundreds of researchers. With access to SPR technology on your own lab bench you can get the high quality data you need to accelerate your research and publish faster.
1. American Society of Clinical Oncology. (2016, October 10). Aging and Cancer. Retrieved May 28, 2018, from https://www.cancer.net/navigating-cancer-care/older-adults/aging-and-cancer
2. Campisi, J. (2013). Aging, Cellular Senescence, and Cancer. Annual Review of Physiology, 75, 685–705. http://doi.org/10.1146/annurev-physiol-030212-183653
3. Aunan, J. R., Cho, W. C., & Søreide, K. (2017). The Biology of Aging and Cancer: A Brief Overview of Shared and Divergent Molecular Hallmarks. Aging and Disease, 8(5), 628–642. http://doi.org/10.14336/AD.2017.0103
