For the first time ever, surface plasmon resonance (SPR) and digital microfluidics (DMF) have come together to revolutionize biomolecular interaction analysis. Nicoya is proud to introduce Alto: the world’s first digital, high-throughput benchtop SPR system. Instead of using physical pumps, valves, and tubes, Alto precisely controls discrete nanodroplets across a grid using electricity. With DMF-powered SPR there is no clogging, contamination or maintenance, problems that plague existing SPR instruments. Reagent/sample storage, fluid handling and fiber optic sensor tips are all contained on a disposable cartridge. This 16-channel system provides quantitative, high-throughput kinetic analysis with end-to-end experiment automation.
In our latest application note, Alto is put to the test by determining the kinetics and affinity of a Protein A – IgG interaction at eight different concentrations. In this blog post, we highlight Alto’s performance in this experiment after diving into the details of digital microfluidics.

What is digital microfluidics?
Digital microfluidics (DMF) manipulates nanoliter-sized droplets based on a concept known as “electrowetting”. Droplets are controlled by applying an insulated electrode voltage on a hydrophobic surface. By turning the voltage on and off in succession across adjacent electrodes, the position of a droplet can be precisely manipulated across an array of electrodes.
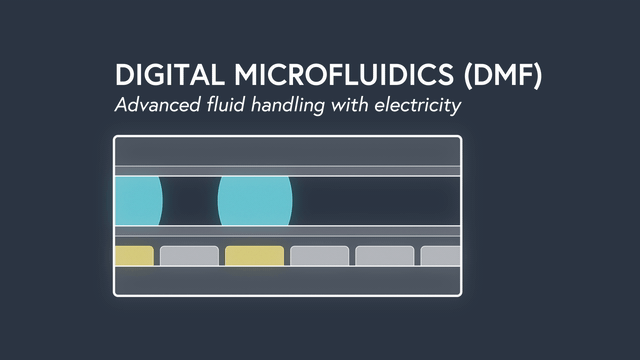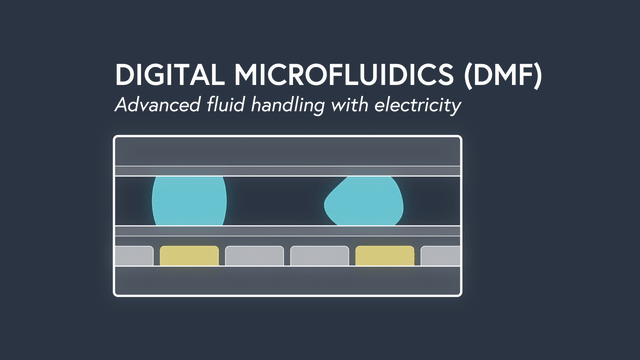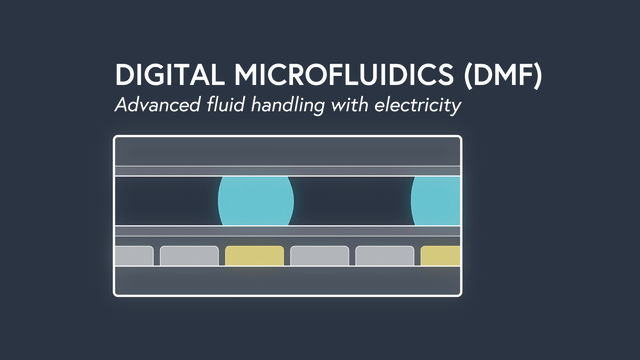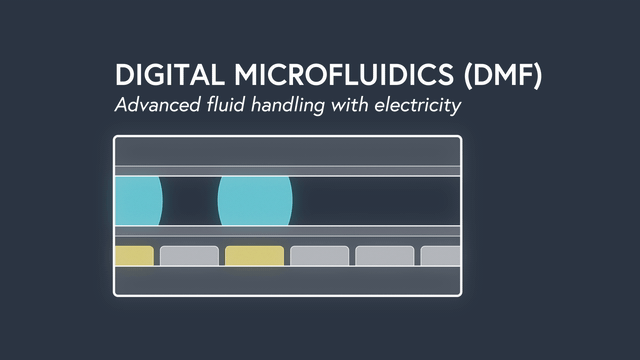
Applying DMF to surface plasmon resonance (SPR) has some incredible results. By precisely manipulating individual droplets sample dispersion is eliminated, the sample volume is significantly decreased to 2ul (up to 500x less compared to traditional SPR), and rise times are the fastest ever recorded (<0.1s). Individual droplets can be split, mixed, merged and dispensed to precisely controlled volumes and concentrations. As a result, serial dilutions are done by the instrument, minimizing assay set up time and preventing mistakes. The entire fluidic system, including wells for sample storage, is contained on a disposable cartridge that is compatible with the standard well-plate form factor. Learn more about the power of DMF.
Kinetic analysis of a Protein A-IgG interaction performed with Alto using 1.125 µL of analyte
In our latest application note, the Alto instrument is set up and a Carboxyl Cartridge is loaded into the instrument. The following reagents are then loaded into the cartridge:
Table 1. Reagent type and volume used for Protein A – IgG experiment
| Reagent | Volume loaded (µL) |
| 900nM IgG | 8.5 |
| 40ug/ml Protein A | 35 |
| 10mM Gly-HCl | 35 |
| Blocking solution | 35 |
| 0.1M EDC | 8.5 |
| 0.1M NHS | 8.5 |
| PBS-T | 105 |
Once the cartridge is loaded, the instrument will automatically complete the pre-designed protocol Protein A is first immobilized onto the eight Active Sensors on the Carboxyl Cartridge automatically. Following the automated protocol, serial dilutions of IgG are then performed on the cartridge to produce eight unique concentrations. The binding of each IgG concentration to Protein A are then analyzed across the eight Active sensors automatically. After completion of the test, the binding curves are fitted to a 1:1 binding model to determine kinetic and affinity constants:
These binding curves demonstrate clear concentration dependence, evident association and dissociation phases, as well as low residuals and errors. The calculated kinetic and affinity constants agree well with other studies of this interaction.
The run time of the entire experiment (including serial dilutions) on Alto was approximately 2 hours, with the majority of the experiment being performed automatically. The intuitive software allowed the user to check on their experiment from their computer anywhere with an internet connection. The DMF-powered system only required a combined total of 1.125 µL of 900nM IgG analyte for all eight concentrations). Visit the Alto product page to learn about the other incredible advancements of Alto, and check out the full experiment here.
