The world’s most user-friendly SPR system can now support regulated workflows
Characterization of biomolecular interactions is an essential part of biologics discovery and development. The Alto Digital SPR system offers unmatched ease of use along with excellent precision and accuracy for binding kinetics measurements and biomolecule quantitation. And now with its GxP suite consisting of software expansion and services, the Alto can support the characterization needed through all stages of the drug R&D.
The features in the Alto GxP software expansion are specifically designed to support compliance with the FDA’s 21 CFR Part 11 guidelines around electronic records.
The GxP software expansion offers robust data protection and integrity with user access controls, password policies, and edit-protected data format and storage. Raw Alto data is saved on the instrument in an edit-protected format and can be recovered post-experiment, regardless of modications made during analysis.
Additionally, the software expansion supports up to three configurable signature levels and reasons. Once signed, results are locked to prevent further modifications.
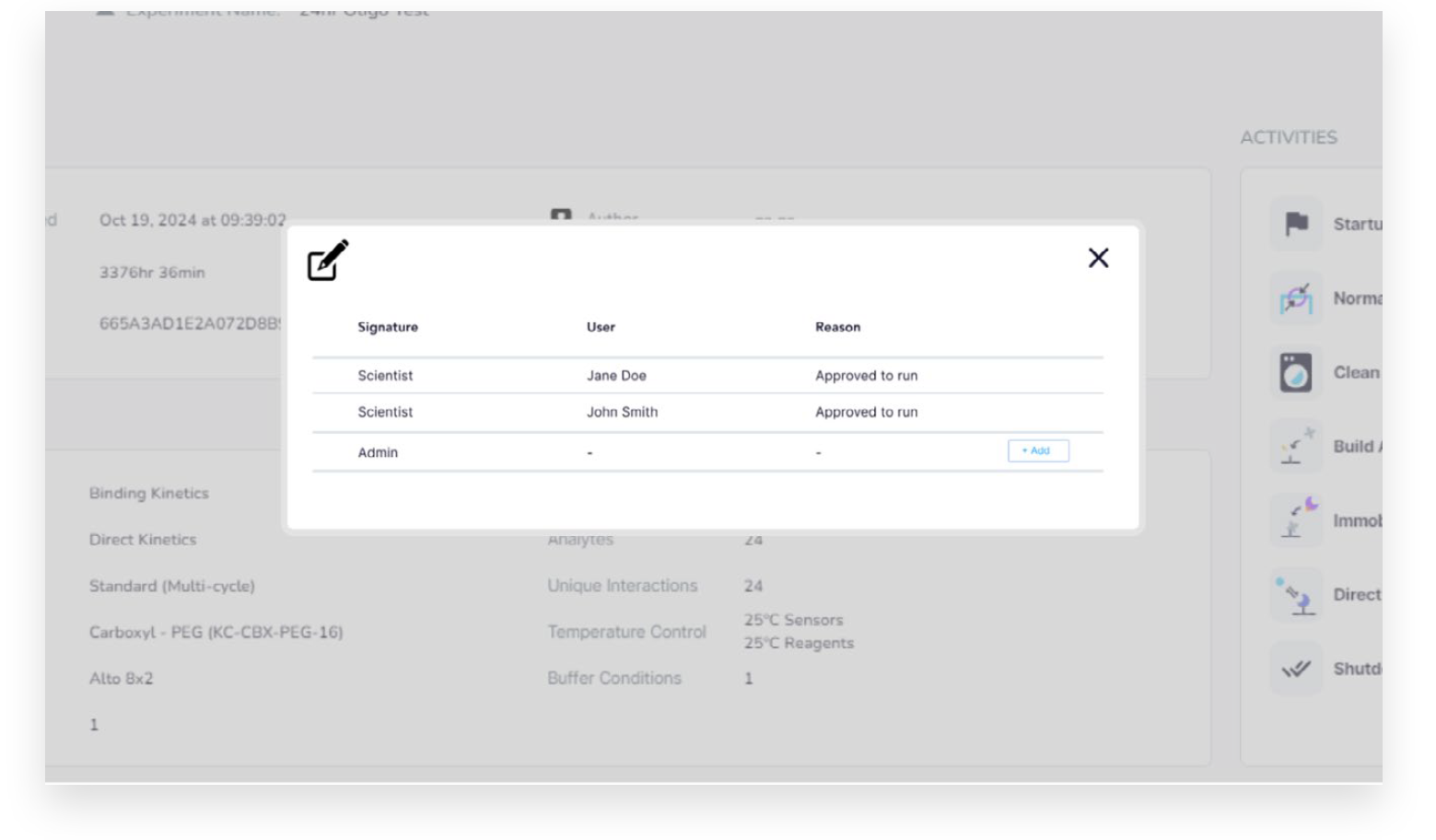
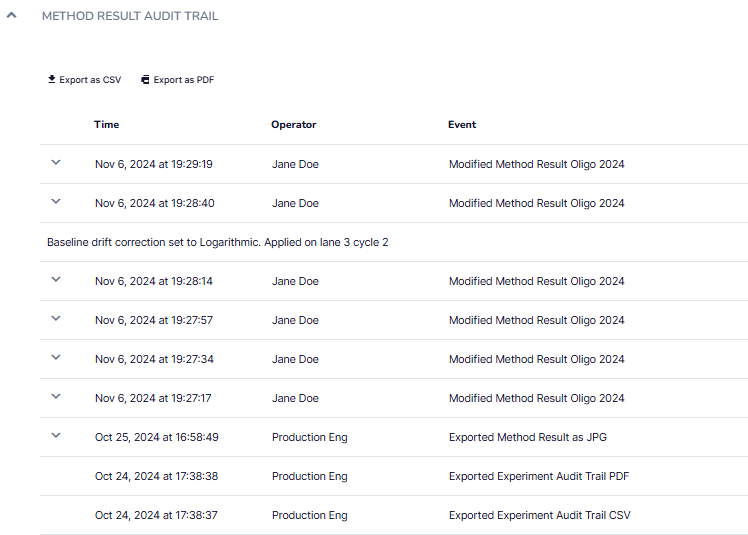
Alto’s GxP software expansion maintains detailed audit trails to ensure full traceability and compliance. Every modification to methods and result files is recorded with timestamps and user information. Audit trails also track system events, such as logins, power cycles, diagnostics, calibration tests, and software updates, providing a complete history to support data integrity and regulatory requirements.
Alto’s GxP software expansion maintains detailed audit trails to ensure full traceability and compliance. Every modification to methods and result files is recorded with timestamps and user information. Audit trails also track system events, such as logins, power cycles, diagnostics, calibration tests, and software updates, providing a complete history to support data integrity and regulatory requirements.

Ensure your Alto system meets specifications with our comprehensive qualification services and kit. These include:
Alto’s qualification options provide confidence in reliable operation and adherence to stringent quality standards.
"*" indicates required fields
"*" indicates required fields
Over 600 researchers worldwide are using OpenSPR™ to get the data reviewers are looking for. Read our brochure to learn more!
"*" indicates required fields
Interested in learning more about how Alto can accelerate your drug discovery? Fill out the form below to download a product brochure.
"*" indicates required fields