Surface plasmon resonance (SPR) can be used to analyze all types of interactions ranging from protein-protein, protein-small molecule, protein-nucleic acid, protein-aptamer, protein-lipid, carbohydrate-protein (carbohydrate-lectin) , carbohydrate-carbohydrate and many more. One area of research is the study of glycoprotein binding kinetics, and many scientists are implementing techniques such as SPR to define the kinetic constants of these types of interactions.
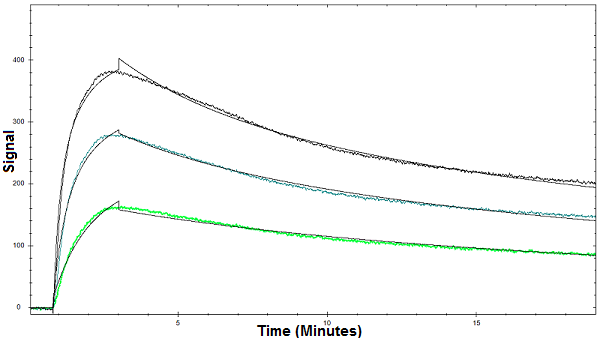
Figure 1. Binding curves of Con A binding to glycan using OpenSPR
Why do we study carbohydrate interactions?
Carbohydrate-protein interactions are important for key processes in biological systems including cell-cell recognition, endocytosis, phagocytosis, signal transduction, and modulating intracellular traffic. Proteins that build to carbohydrates are called lectins and are found in animals, plants and microorganisms. Glycoproteins and glycolipids are proteins and lipids that have oligosaccharide chains (glycans) attached to them, and these are the carbohydrates that lectins bind to. The carbohydrates are attached to proteins during a cotranslational or posttranslational modification called glycosylation. The glycan chains are important for recognition processes in biological systems.
The cell membrane: Carbohydrates attached to proteins and lipids are important for cell recognition
Why do researchers use SPR to characterize carbohydrate-protein interactions?
Determining the binding kinetics and affinity of carbohydrate-protein interactions is important for understanding how lectins modulate signal processes in biological systems. Techniques such as quartz crystal microbalance (QCM), enzyme-linked lectin assays (ELLAs), NMR in conjunction with isothermal calorimetry (ITC) and fluorescence spectroscopy have been used to study these interactions. Many of these techniques require complex labelling steps or expensive, complex instrumentation. In recent years, many researchers have turned to benchtop surface plasmon resonance (SPR) to characterize their carbohydrate-lectin interactions as a user-friendly and low maintenance solution. SPR is a label-free technology which allows researchers to quantitatively analyze binding between two biomolecules. SPR technology allows us to determine the kon, koff and KD of interactions, providing deeper insight into binding events compared to other techniques that only give endpoint measurements.
Example of a lectin (carbohydrate binding protein) interaction analyze using OpenSPR™
Below is an example of how OpenSPR™ was used to characterize the binding kinetics of a lectin-glycan interaction. OpenSPR sensor chips were activated with a glycan coating and the interaction between the glycan and concanavalin A (con A) was measured. The glycan coating immobilized on the sensor chips is composed of oligosaccharide chains that you would find attached to a glycoprotein in a biological system. Con A is a lectin that binds to a number of sugars, glycoproteins, and glycolipids. Three concentrations of Con A were tested and the binding kinetics and affinity were determined using a bivalent binding model. This illustrates the value of surface plasmon resonance analysis in such applications as the strength of the interaction between the glycan and lectin can be easily determined. You can find the full study here.

Figure 1. Binding curves of Con A binding to glycan using OpenSPR
M.J. Linman et al. “Surface Plasmon Resonance Study of Protein-Carbohydrate Interactions using Biotinylated Sialosides.” Anal Chem, Vol. 80, pp. 4007-4013, 2008.
