Alto’s unmatched ability to seamlessly characterize mAbs make it the optimal fit for biologics developers. Read our latest application note on digital SPR analysis of the interleukin-6 receptor (IL-6R) to learn more.
A decade after it was first introduced to the market, the drug tocilizumab gained attention once again in 2021 for being issued emergency use authorization (EUA) by the FDA for the treatment of COVID-19 in certain hospitalized patients. Tocilizumab, or Actemra, is a humanized monoclonal antibody (mAb) that reduces inflammation by blocking the interleukin-6 receptor, and is currently being used for the treatment of chronic inflammatory diseases.
Therapeutic mAbs are one of the fastest growing classes of therapeutics for a wide range of diseases, and interleukins and their signaling pathways are preeminent targets for treatment due to the vast number of problems presented by their improper regulation. Its authorization as a treatment for COVID-19 demonstrated the wider potential of IL-6 as a therapeutic strategy for other diseases, highlighting the importance of further characterizing the IL-6 mechanism of action and its therapeutic applications.
Surface plasmon resonance (SPR) is a powerful tool for analyzing the binding interactions associated with signaling proteins, their receptors and related effectors. In our latest application note, we demonstrate how Alto™ Digital SPR overcomes the challenges of traditional binding techniques in characterizing interleukin-6 receptor (IL-6R) for therapeutic mAb development.
Table of contents
- Interleukin-6 mechanism of action (MoA)
- Therapeutic mAbs against interleukins
- Accelerate mAb development with digital SPR
- Characterization of IL-6R with Alto
Interleukin-6 mechanism of action (MoA)
Interleukins, a type of cytokine, are a group of proteins involved in critical immune cell functions such as proliferation, differentiation, and adhesion. They are of particular importance in coordinating immune-related stresses such as inflammation.
Once secreted by cells, they travel to their target cell where they bind to their receptor, triggering their respective downstream cascades. Playing such critical roles in immune and stress responses, dysregulation of interleukins can result in debilitating conditions such as autoimmune diseases and cancer.
Interleukin-6 (IL-6) is a well known pro-inflammatory cytokine expressed in response to various environmental stress factors, such as infections or damage to cell tissue, to trigger the appropriate defense mechanisms. However, improper regulation of IL-6 and its receptor, IL-6R, can result in the development of several diseases due to the stimulation of inflammatory and auto-immune processes.
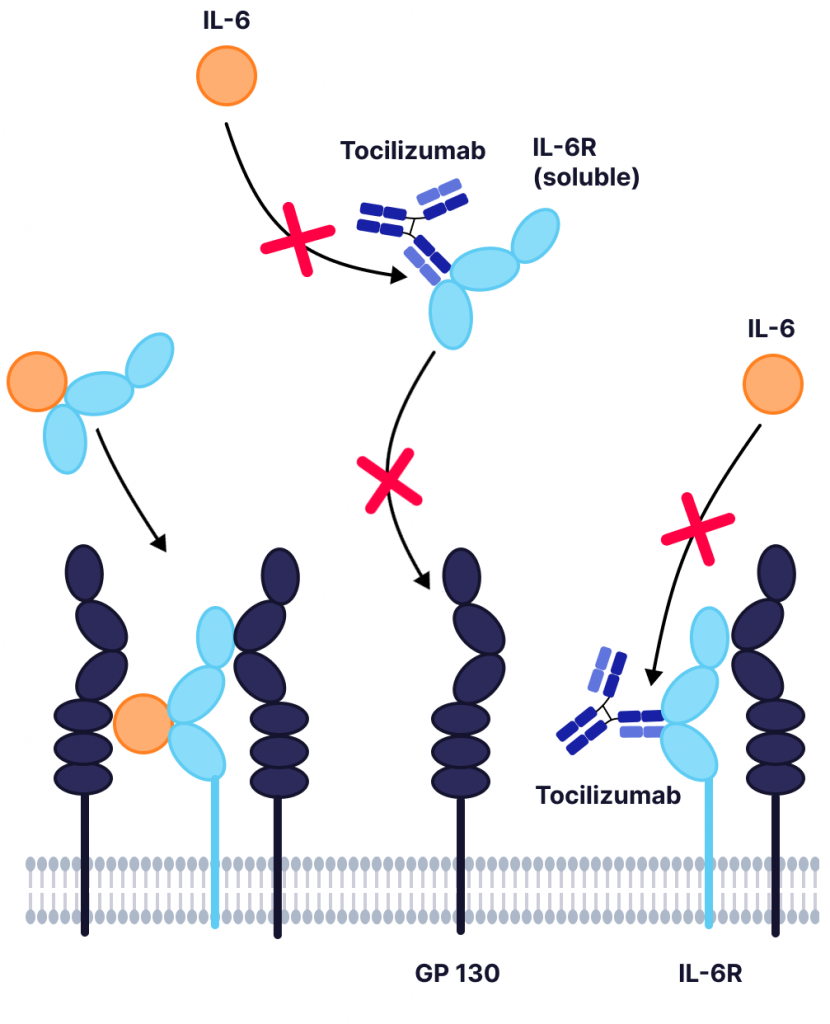
Blockade of the IL-6 signaling pathway has become a target for treating chronic inflammatory conditions where IL-6 is implicated. Tocilizumab is an FDA approved mAb with high binding affinity for IL-6R, allowing it to outcompete IL-6 and inhibit the resulting receptor–signaling system (Figure 1). It is commonly used to treat rheumatoid arthritis and systemic juvenile rheumatoid arthritis.
Figure 1: Inhibition of IL-6 signaling by tocilizumab. IL-6 binding to IL-6R or the soluble IL-6R (sIL-6R) causes the dimerization of gp130 and triggers a downstream signaling cascade. Tocilizumab binds to the IL-6R (or sIL-6R), preventing the binding of IL-6, which mediates IL-6 trans-signaling and reduces the chance of a cytokine storm.
Therapeutic mAbs against interleukins
Devising successful monoclonal antibody therapeutics requires the identification or design of a panel of candidates, and their subsequent evaluation based on criteria such as selectivity, specificity, affinity, and the ability to dysregulate the intended signaling cascade. Surface plasmon resonance (SPR) is a powerful tool that meets these needs and is well-suited for analyzing the binding interactions associated with signaling proteins, their receptors and related effectors at all stages of the drug development process. In particular, therapeutic monoclonal antibodies are highly compatible with SPR due to their large size and high affinity binding.
Compared to more time-consuming and semi-quantitative techniques, such as ELISAs and western blots, SPR offers a more efficient and information-rich method for screening and characterizing new therapeutic candidates. In addition to providing affinity data, the real-time nature of SPR enables the analysis of binding kinetics, providing deeper insights into a drug’s clinical efficacy, safety, and duration of action. It also eliminates the need for labels, simplifying the assay design process and reducing hands-on time with the system.
Accelerate mAb development with digital SPR
Alto, a high-throughput SPR platform enabled with digital microfluidic (DMF) technology, offers a number of advantages for characterizing monoclonal antibody drugs and their targets.
With the ability to discreetly control nanoliter-sized droplets of each ligand and analyte, binding assays are simple to implement and provide novel insights into therapeutic performance, while reducing consumption of precious samples by up to 200X. In addition, Alto’s 16 independent channels allow for simultaneous analysis of multiple targets in many different assay formats, while significantly reducing hands-on time with complete assay automation.
In a recent application note, we demonstrated the applicability of Alto in developing new monoclonal antibodies that target IL-6 by characterizing IL-6R binding through several experimental approaches.
Read the full application note here.
Characterization of IL-6R with Alto
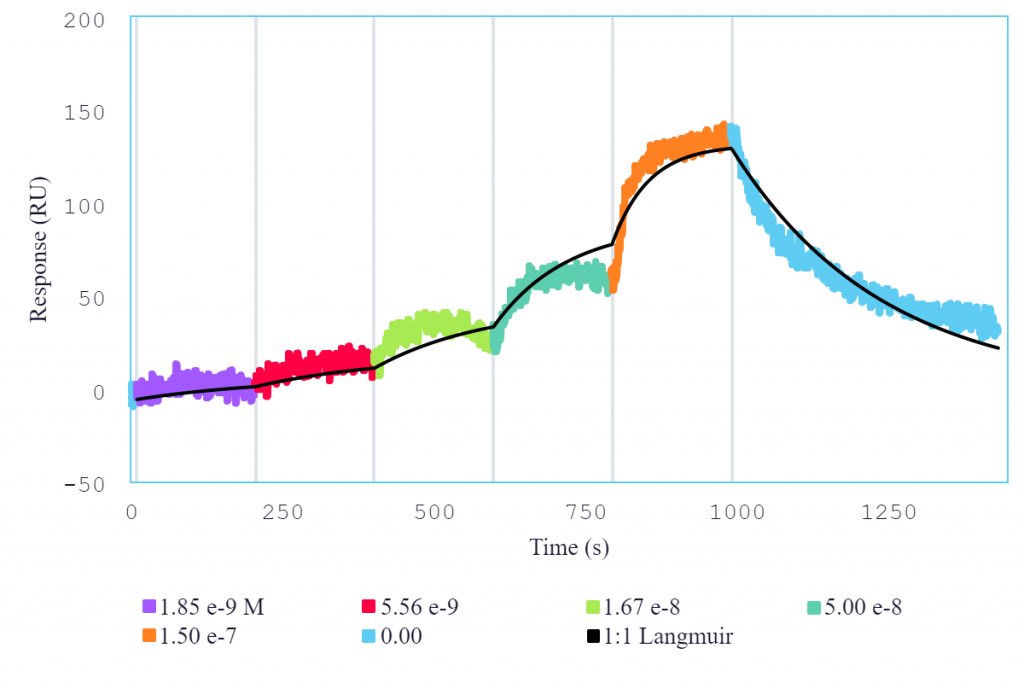
An analysis of multiple interactions of different molecules with IL-6R using Alto demonstrated the platform’s ability to characterize a wide range of affinities while providing comparable data to conventional SPR systems. Single-cycle kinetics (SCK) was used to determine the interaction between IL-6R and IL-6, both for the binding of IL-6 to IL-6R immobilized on a streptavidin surface, as well as for IL-6R binding to directly immobilized IL-6 (Figure 2). SCK was also used to characterize the interaction between tocilizumab and IL-6R.
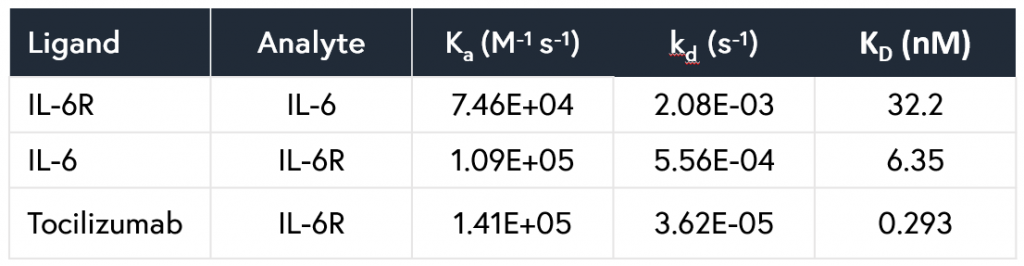
The resulting affinity and kinetics data were determined to be in close agreement with previously determined SPR data. In addition, all data points were generated from a single consumable in under 5 hours, while requiring just 30 minutes of initial setup time and a total of 2 μL per unique sample. All resulting kinetic and affinity values are summarized in Table 1.
Figure 2: Single-cycle kinetics of IL-6 (analyte) binding to immobilized IL-6R (ligand) on Alto. Analyte was titrated from 1.85 nM to 150 nM. Black curve is the Langmuir 1:1 binding fit model analyzed in the Nicoya Analysis Software.
Table 1: Kinetic parameters measured for the interactions of the IL-6/IL-6R and IL-6R/tocilizumab systems using Alto.
With IL-6 being such a widely investigated target for new mAb therapeutics, tools that can evaluate the wide network of interactions involved in its signaling pathways are critical to developing highly-targeted drugs. This characterization study demonstrates Alto’s ability to simultaneously characterize multiple interaction systems with varying affinities, while providing comparable data to conventional SPR systems.
Alto’s unmatched capabilities in accelerating mAb development provide the optimal fit for biologics developers striving to get new therapeutics to market without compromising on efficiency and time. To learn more about how Alto can accelerate your mAb development pipeline, check out the full application note, or get in touch with our team for an in-person demonstration!