The world’s most user-friendly SPR system can now support regulated workflows!

Achieving FDA approval for a new drug is hard, but getting the binding assay data you need for it doesn’t have to be. With only 2 µL of sample needed for a full binding kinetics analysis, 16 parallel channel throughput, and low maintenance needs, Alto and its new GxP suite can streamline your affinity measurement needs through all stages of the drug development process. Here’s a look at the various features included in the Alto GxP Suite™.
Alto’s GxP software expansion comes as a perpetual license with unlimited seats which includes all the features needed to support you in achieving GxP compliance:
- Audit trails
- Enhance data integrity
- Access and authentication controls
- Electronic signatures
- Data backups
The GxP expansion was built with compliance and ease of use in mind: all user and system configurations can easily be set in the admin panel, where data backups and other useful system files can also be accessed.
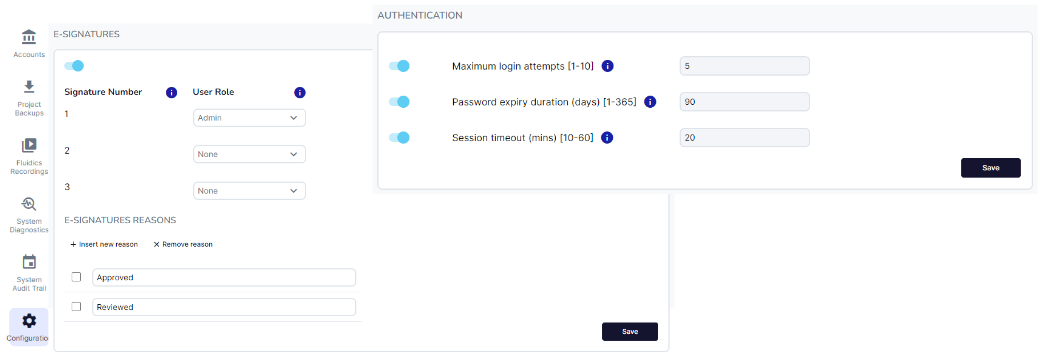
Our file workflows are designed to ensure integrity and traceability. All creation, deletion and modification actions are recorded in audit trails, and file integrity is maintained through a rigorous publication and electronic signature process, which enforces reviews and sign-offs before running methods, and finalizing analysis.
In addition to the software expansion, Nicoya offers qualification kits, and preventive maintenance services to ensure your instrument remains in optimal condition and consistently produces reliable data.
With its GxP suite, Alto helps you generate reproducible, traceable and trustworthy affinity data, so you can bring life-changing therapeutics to market.
Click here for more detailed information on the Alto GxP Suite or reach out support@nicoyalife.com to learn more.
