Overview
Characterizing the binding and kinetics of antibody antigen interactions in serum is critical for advancing the development of vaccines, diagnostics, and other biotherapeutics. Screening these interactions without extensive purification is highly desirable as it reduces cost and hands-on time. In traditional label-free binding assays, serum samples need to be diluted and analyzed using standard running buffers such as PBS. In this application note, we demonstrate a novel approach using Alto™ Digital SPR™ for measuring binding kinetics and affinity of antibody-antigen interactions that allows experiments to be conducted in serum without the need for dilution or purification.
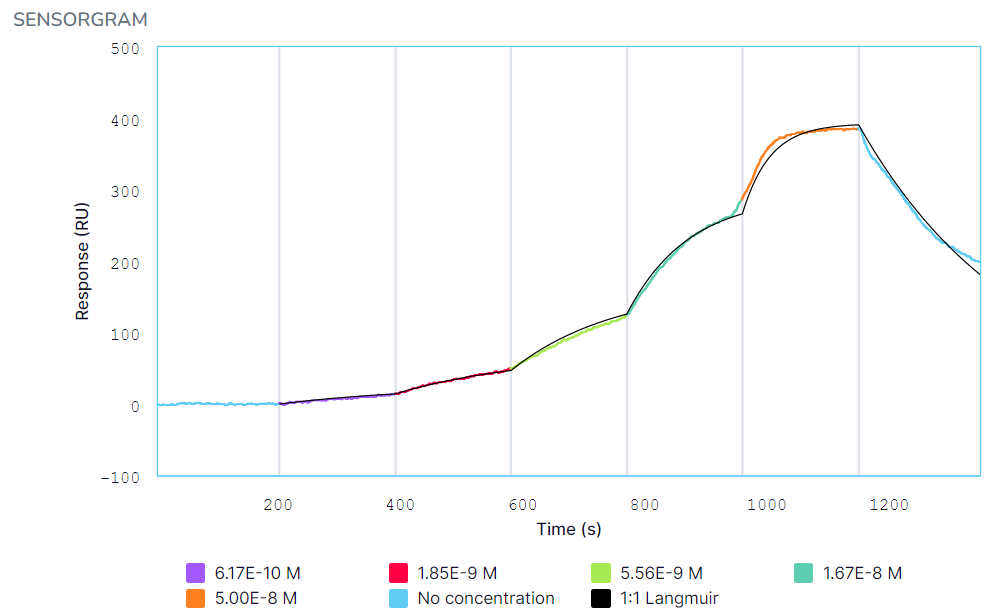
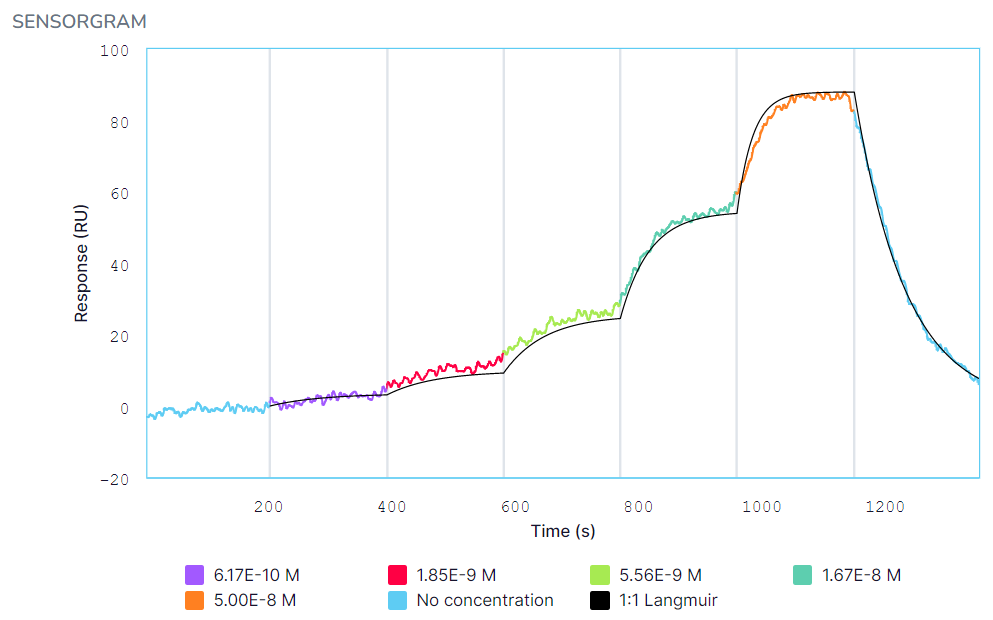
Kinetic fits for HuCAL binding to eGFP in (A) PBS-T and (B) 100% serum + 0.1% Tween 20. The HuCAL analyte was titrated from 0.617 nM to 50 nM, followed by a dissociation in PBS-T or serum. Black curves represent the Langmuir 1:1 binding fit model generated by the Nicosystem software.