Before we dive deep into sensors, make sure that you’re familiar with Surface Plasmon Resonance (SPR) by reading our blog post: What is SPR?
SPR is a label-free technique that enables you to get in depth information on how two biomolecules are interacting. This is done on a sensor inside of an instrument such as the OpenSPR or Alto. SPR instruments are primarily used to measure binding affinity and kinetics, and are becoming increasingly more affordable and accessible as researchers realize the value that SPR data can bring to their research.
In a typical SPR assay, one of your binding partners must be immobilized onto the surface of the sensor. The immobilized molecule is referred to as the ligand. The sensor is pre-functionalized with a specific surface chemistry to make immobilization of your ligand easier, so that your other binding partner (referred to as the analyte) can successfully interact with it and your system can output valuable data on how these two molecules are interacting.
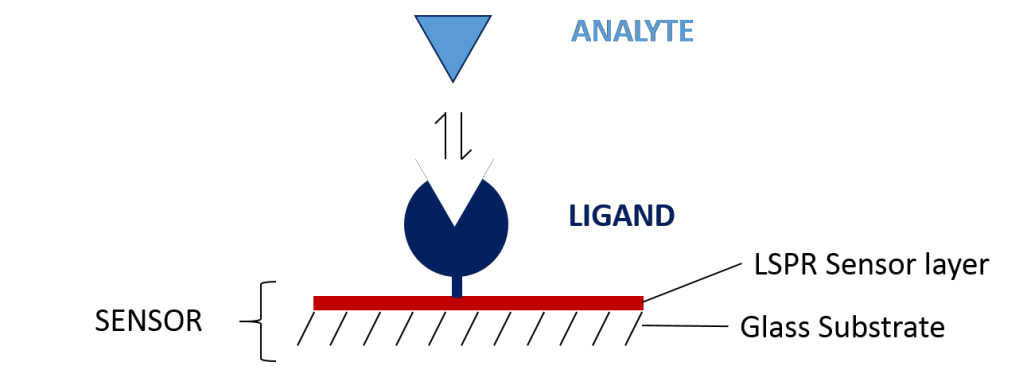
Before getting started on your experiment, you must first determine which sensor will be best for your chosen application. This largely depends on your ligand, as there are three major ways of immobilizing ligands on the sensor: covalent coupling, capture coupling and hydrophobic capturing. This guide will walk you through these immobilization techniques so you can determine which sensor will be best suited for your experiment!
The Types of SPR Sensors
Sensors consist of a glass substrate, gold nanoparticles, and a functional chemical coating that allows the ligand to be immobilized to the sensor surface. Choosing the right sensor depends largely on the characteristics of your ligand: Is it tagged or untagged? Does it have any functional groups? Is it an IgG based antibody? Is it a lipid or a protein reconstituted into a liposome?
Regardless of the sensor chemistry, there are generally inherited benefits to using SPR sensors, such as reusability. The number of times you can reuse a sensor with the ligand attached depends on the stability of the ligand. Here is our list of sensors at Nicoya:
Covalent coupling sensors:
- Carboxyl – for coupling to activated amine groups on the ligand
- Amine – for coupling to activated carboxyl groups on the ligand
- Gold – no surface chemistry; for custom surface chemistry development and direct immobilization of ligands with thiol groups
Capture coupling sensors:
- Biotin-Streptavidin Sensor Kit – for coupling to biotinylated ligands
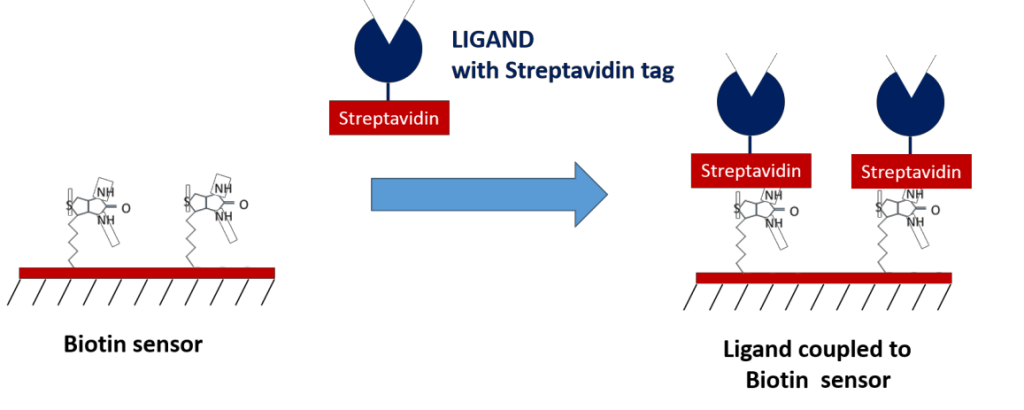
- Biotin – for coupling to streptavidin-tagged ligands
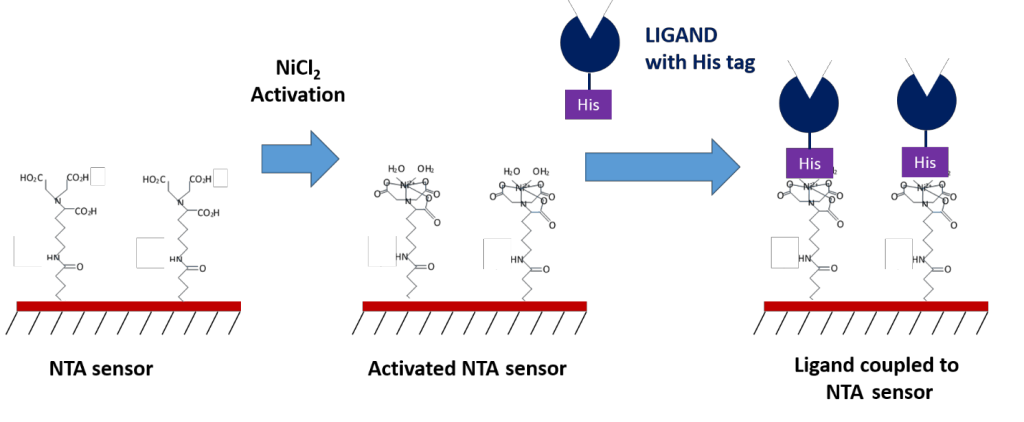
- NTA – for coupling to his-tagged ligands
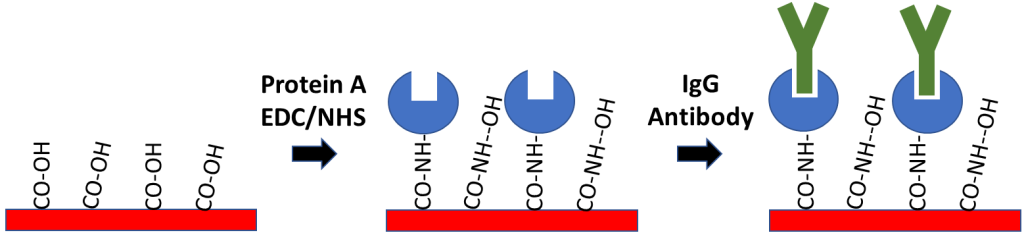
- Protein A Sensor Kit – for coupling to IgG based antibodies
Hydrophobic capture sensors:
- Liposome – for coupling liposomes
- Hydrophobic – for lipid monolayer formation
The chart below offers a convenient summary of which sensor to use for your applications:
| Sensor: | For the immobilization of: |
| Carboxyl | Any amine group using EDC/NHS coupling |
| Amine | Any carboxyl group using EDC/NHS coupling |
| Gold | Non-functionalized and perfect for custom surface chemistry development and targets with thiol groups |
| Biotin-Streptavidin Sensor Kit | Biotinylated ligands |
| Biotin | Streptavidin-tagged ligands |
| NTA | Histidine-tagged targets |
| Protein A Sensor Kit | IgG based antibodies |
| Liposome | Liposomes/membrane proteins |
| Hydrophobic | Lipid monolayer formation |
Covalent Coupling – SPR Sensors
Covalent coupling irreversibly attaches the ligand to the sensor’s surface using a standard covalent interaction.
Advantages:
- Reduces ligand dissociation
- Maintains the binding capacity throughout the experiment(s) (provided that the ligand is stable)
- Compatible with most biomolecules, which minimizes the need for chemical or genetic modifications to the ligand
1. Carboxyl Sensors
Carboxyl sensors require EDC/NHS chemistry to activate the functional groups on the sensor surface, which can then bind to the ligand’s available amine groups or lysine residues. To help with this, we’ve put together a well-crafted activation kit which contains all reagents necessary to perform this coupling and has been optimized for best performance.
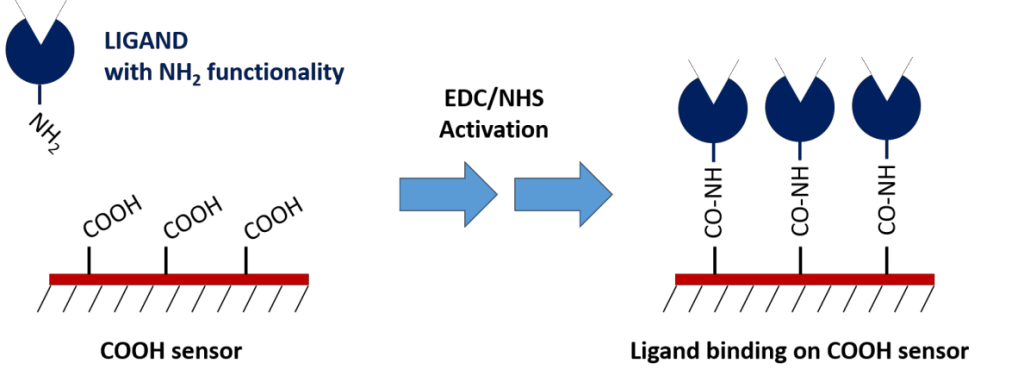
The greatest benefit of this coupling method is that it’s relatively straightforward to perform in addition to being consistent and stable. The main limitation of this method is that the orientation of the ligand cannot be controlled because the coupling site cannot be specified. Another limitation is that covalent immobilization of the ligand can, in some cases, affect the analyte-ligand interaction. If these issues become apparent, you can opt for a capture coupling method.
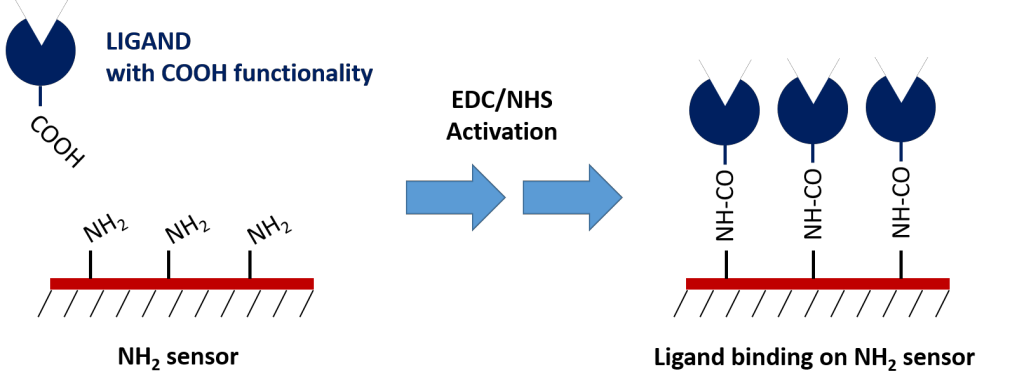
2. Amine Sensors
Similar to Carboxyl sensors, Amine sensors require EDC/NHS chemistry to form the covalent bond between the ligand and the sensor surface. However, the EDC/NHS activation modifies the available carboxyl groups on the ligand, potentially affecting it’s binding activity. Thus, Carboxyl sensors are generally preferred for protein ligand immobilization. Amine sensors are most commonly used for immobilization of ligands containing a carboxylic acid tag that does not participate in the binding interaction.
3. Gold Sensors
Gold sensors are used for direct immobilization of ligands containing a thiol group. The thiol group, either native to the ligand or specifically introduced specifically as a tag, will spontaneously form a strong bond with the gold surface. As the native gold surface is inherently sticky, an important step is to block any uncoated gold surface. To do this, short thiolated PEG molecules and BSA can be used after the immobilization of the ligand to prevent non-specific binding of the analyte.
Another reason for choosing a Gold sensor is that it is a blank slate. This means that you have the flexibility to develop your own custom sensor chemistry for unique and challenging applications. This also provides an ideal platform for providing a rich teaching environment in undergraduate labs.
Capture Coupling – SPR Sensors
Capture coupling occurs when the ligand is immobilized through a non-covalent interaction with an intermediate capture molecule, which is typically covalently coupled to the surface.
Advantages:
- No ligand modification required in some cases
- Ligand orientation is uniform and homogenous
- Capture molecule is specific to the ligand
- Ability to reuse and regenerate the same sensor surface
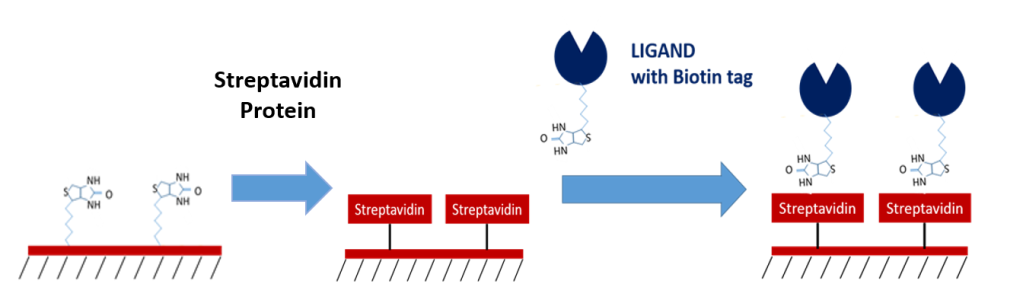
4 & 5. Biotin/Streptavidin
Biotin and streptavidin are known to have one of the highest affinities of any biomolecular interaction, making them an excellent method of immobilization for ligands tagged with streptavidin or biotin. Their high affinity makes them extremely reliable as intermediate capture molecules, providing benefits such as the ability to withstand regeneration conditions which resemble the nature of covalent capturing, no background off-rate which may affect your data, and the ability to control for ligand orientation. If you are able to tag your molecules with biotin and/or streptavidin, these sensors can be ideal for your experiments!
6. NTA Sensors
Another commonly used compound for capture coupling is nitrilotriacetic acid (NTA), which can capture his-tagged ligands. This technique provides a convenient method of immobilization for those who use poly-histidine (His-6) tags for purification purposes. An advantage to NTA sensors is that the surface can often be reused, as the his-tagged ligand can be easily removed using chelating agents like EDTA. However, depending on your application, the lower bond strength of the coupled ligand can sometimes be a disadvantage as it may cause the ligand to slowly dissociate from the sensor surface over time, or result in unwanted ligand removal during regeneration. If your ligand is his-tagged, NTA sensors are a convenient and versatile option to explore.
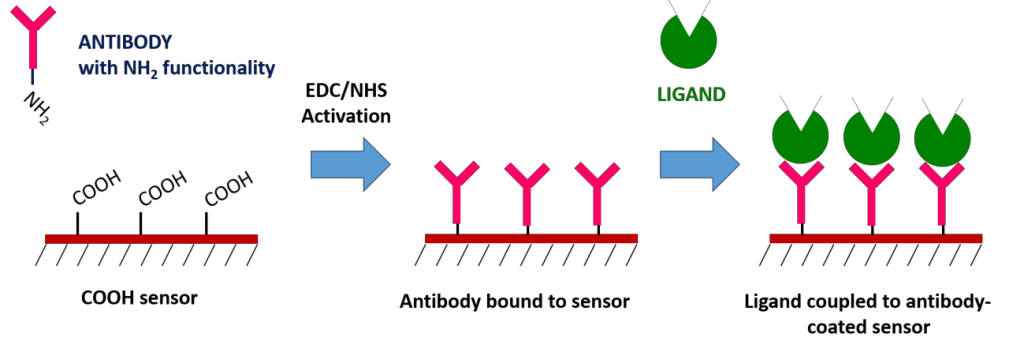
7. Antibody Capture – Carboxyl Sensors
High affinity antibodies are available for many proteins and can be used for specific ligand capture. This is done on a traditional Carboxyl sensor by first immobilizing the antibody, and then using the immobilized antibody to capture the ligand of interest. The biggest advantage of the antibody coupling method is that it does not affect ligand activity and allows for specific ligand orientation. You can also use this method to capture ligands from complex samples, which would allow you to confirm the presence of a suspected protein within a crude sample. However, keep in mind that regeneration will not remove the antibody from the surface.
8. Antibody Capture – Protein A Sensor Kit
Protein A Sensors offer a method of capture coupling for immobilization of antibody ligands. Protein A is used to capture the Fc region of IgG antibodies, allowing for directional immobilization of the antibody where the variable region is oriented away from the sensor surface. In order to prepare this sensor, Protein A is covalently coupled to a Carboxyl sensor after EDC/NHS activation to create a Protein A functionalized surface. The main advantage of this method is that it allows for specific ligand orientation while creating a stable attachment of your antibody ligand to the sensor surface. If you’re considering working with an antibody ligand, our Protein A Sensor Kit contains all the reagents necessary to prepare your surface.
Hydrophobic Capturing – SPR Sensors
These sensors are specifically designed for use with lipids, membrane systems, or very hydrophobic proteins.
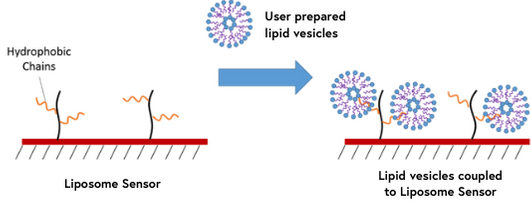
9. Liposome Sensors
Liposomes are a widely used tool for studying lipid-lipid and protein-lipid interactions as they can provide a simple model for looking at the otherwise complex biophysical properties of cellular membranes. Liposome sensors contain lipophilic groups which are capable of capturing lipid vesicles. This method of immobilization is valuable for studying interactions of membrane proteins and other membrane systems that would normally be challenging with traditional binding techniques.
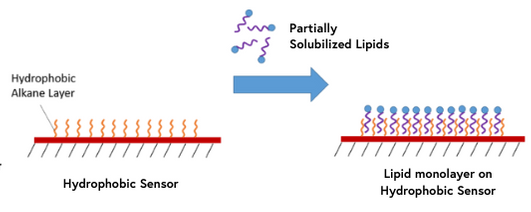
10. Hydrophobic Sensors
Hydrophobic sensors are coated with a hydrophobic layer of long-chain alkane molecules which allow for the immobilization of lipid molecules for interaction analysis. Partially solubilized lipids will self-assemble into a monolayer with hydrophilic groups positioned away from the surface. If you are looking at lipid-related interactions, our hydrophobic sensors may provide the ideal conditions for your ligands.
We hope this guide has helped you determine which sensor is best for your application. If you are looking to purchase SPR sensors, we recommend visiting our sensor store. One thing to keep in mind is that our sensors have an estimated shelf life of 6 months in proper storage conditions for obtaining the highest quality SPR data. To learn more about SPR sensors, speak to an application scientist today!
