Researchers at Duke University led by Dr. Michael Tadross made a significant contribution to the field of neuroscience by introducing a cutting-edge method called DART.2 (Drug Acutely Restricted by Tethering). DART.2 allows for highly targeted drug delivery to specific types of neurons in the brain, which has the potential to revolutionize how researchers study neural connections and treat neurological diseases. This work was recently published as an article in Nature Methods.1
Table of contents
The challenge of precision in drug discovery
The evolution of DART: From concept to DART.2
How DART.2 works
Validating DART.2 with Nicoya Alto digital SPR
Implications and future directions
The challenge of precision in drug delivery
In the realm of neuroscience, drugs are crucial tools for exploring the intricate networks that govern brain function. However, a significant challenge has always been the lack of precision in delivering molecules to the right location. When a drug binds to specific receptors, it doesn’t just affect the intended target cells; it can influence millions of other cells expressing the same receptor. This off-target activity often leads to side effects and complicates the understanding of the drug’s impact on key neural signals. .
The evolution of DART: From concept to DART.2
Dr. Tadross and his team set out with a simple yet profound goal: to ensure that a drug works precisely where it’s needed in the brain. This led to the development of the first-generation DART system in 2017.2 DART could deliver drugs to specific brain cells by using a protein selectively expressed on the surface of target neurons. When a drug was introduced, it was captured by this “beacon” protein, creating a concentrated effect around the target cells. The first-generation DART could increase the drug concentration in a specific brain region 30-fold within minutes.
This year, the team introduced DART.2, a vastly improved version of the technology that can amplify the local drug concentration by up to 3,000-fold within just 15 minutes.
How DART.2 works
DART.2 exploits the HaloTag labeling system to covalently tether a drug to its target cell. Target neurons selectively express the HaloTag protein on their surface that irreversibly captures the bifunctional HaloTag ligand-drug conjugate. The HaloTag protein-ligand interaction greatly increases the concentration of the drug in the proximity of the target cell and only allows the drug to bind receptors nearby the HaloTag protein. This results in selective binding of the drug to receptors of target neurons, reducing off-target effects.
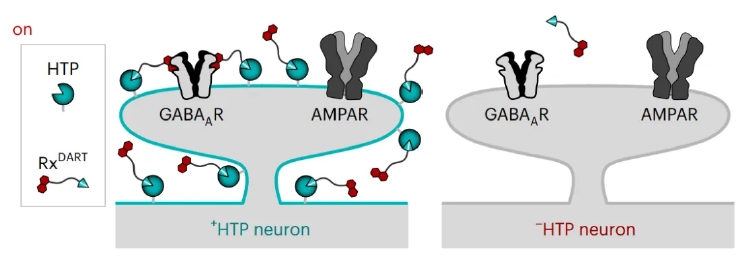
Figure 1. Diagram of the DART system. HaloTag protein (HTP) is selectively expressed on the target neuron and binds to RxDART, a bifunctional molecule composed of the HaloTag ligand (HTL) and the drug of interest. The HTP-HTL interaction increases the local concentration of the drug allowing the drug to bind to nearby receptors on that target neuron. Image taken from Shields et al.1
The targeting accuracy of DART.2 hinges on the binding strength between HaloTag ligand and the HaloTag protein on the neurons. Through meticulous design and testing, the researchers identified a new candidate HaloTag ligand, dubbed HTL.2, which exhibited a higher capture efficiency in neuronal cells compared to its predecessor, HTL.1. Remarkably, even at low concentrations (30 nM), HTL.2 maintained a high capture level.
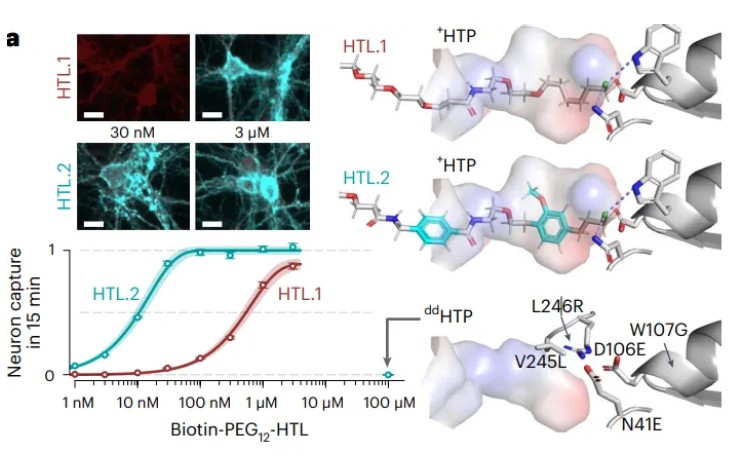
Figure 2. Data comparing the interaction of HTL.1 and HTL.2 with HTP. Top left, neuronal CC50 assay where HTP expression is shown in red and HTL.1/HTL.2 in cyan, highlighting the increased capture of HTL.2 compared to HTL. 1 at 30 nM. Bottom left, quantification of neuronal CC50 assay. Right, structural models of the HTL.1 and HTL.2 interaction with HTP. Image taken from Shields et al.1
Validating DART.2 with Nicoya Alto digital SPR
To validate the binding rates of HTL.1 and HTL.2 with HTP, the researchers turned to Nicoya’s Alto digital Surface Plasmon Resonance (SPR) technology. SPR is considered the “gold standard” for detecting molecular interaction dynamics in vitro. The Nicoya Alto system provided several advantages:
- Micro-quantitative samples needed: Only 2 μL of sample was required to obtain complete binding kinetics detection at five concentration points.
- Fluidics-free design: The integration of the SPR sensor, sample, and flow system into a disposable cartridge eliminates maintenance costs.
- High throughput: The cartridge’s 16 channels allow for high-throughput SPR detection.
- Automation: Sample dilution, detection, and data analysis are highly automated, streamlining the entire process.
Using Nicoya Alto, the researchers discovered that HTL.2’s binding rate to HTP was approximately 45 times greater than that of HTL.1, corroborating the results obtained from intracellular experiments.
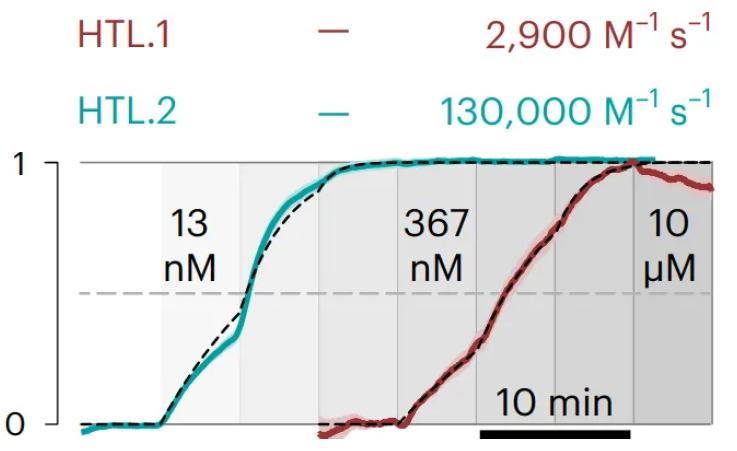
Figure 3. Alto digital SPR data showing HTP (ligand) binding HTL.1 and HTL.2 (analytes). The association rate constant for each HTL is shown above the sensorgram. Image taken from Shields et al.1
Implications and future directions
The enhanced cell-targeting accuracy of DART.2 opens new possibilities for drug research in living animals. For example, the researchers conducted validation experiments using gabazine, a drug that prevents the neurotransmitter GABA (γ-aminobutyric acid) from binding to its receptors. Gabazine is known for its ability to induce epilepsy at low concentrations, making it a challenging drug to study. DART.2’s precision targeting made it possible to optimize both the method and effects of gabazine administration in animal models.
The DART.2 system represents a significant leap forward in precision drug delivery, with broad implications for neuroscience research and the treatment of neurological disorders.
To learn more about the Nicoya Alto digital SPR, please visit our website!
References
- Shields BC, Yan H, Lim SS, et al. Dart.2: Bidirectional synaptic pharmacology with thousandfold cellular specificity. Nat. Meth. 2024;21(7):1288–1297. doi:10.1038/s41592-024-02292-9
- Shields BC, Kahuno E, Kim C, at al. Deconstructing behavioral neuropharmacology with cellular specificity. Science. 2017;356(6333). doi: 10.1126/science.aaj2161