Overview
Biomolecular interaction assays are often conducted at subphysiological temperatures due to equipment limitations, however, temperature significantly influences biomolecular behavior, structure, function, and interactions. For certain applications, such as vaccine development, determining binding kinetics at physiological temperature is critical. In this application note, we demonstrate the ability of the Alto™ Digital SPR™ platform to evaluate the temperature dependence of binding kinetics for an Influenza A hemagglutinin–antibody pair by measuring binding at both 25°C and 37°C. These results demonstrate Alto’s ability to capture physiologically relevant kinetics and highlights the importance of measuring at physiological temperatures during development.
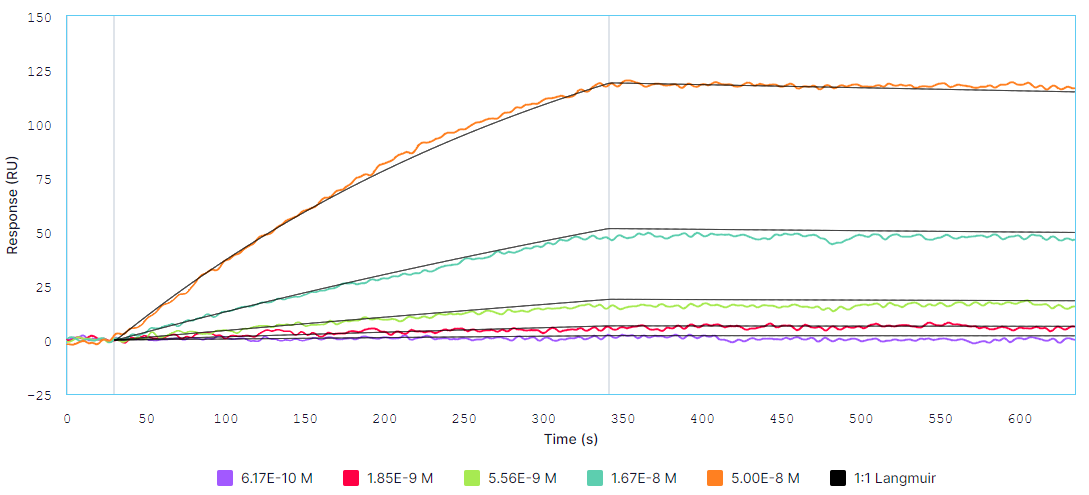
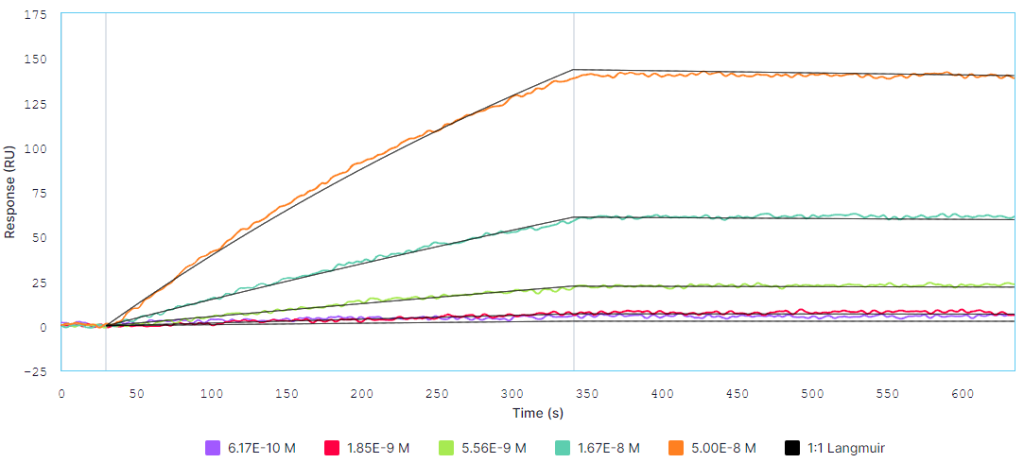
Multi-cycle kinetics of H5N1 HA (analyte) binding to captured RM02 (ligand) on Alto at A) 25°C and B) 37°C. The analyte was titrated from 0.617 nM to 50 nM. Black curves are the Langmuir 1:1 binding fit model generated by the Nicosystem software.