Overview
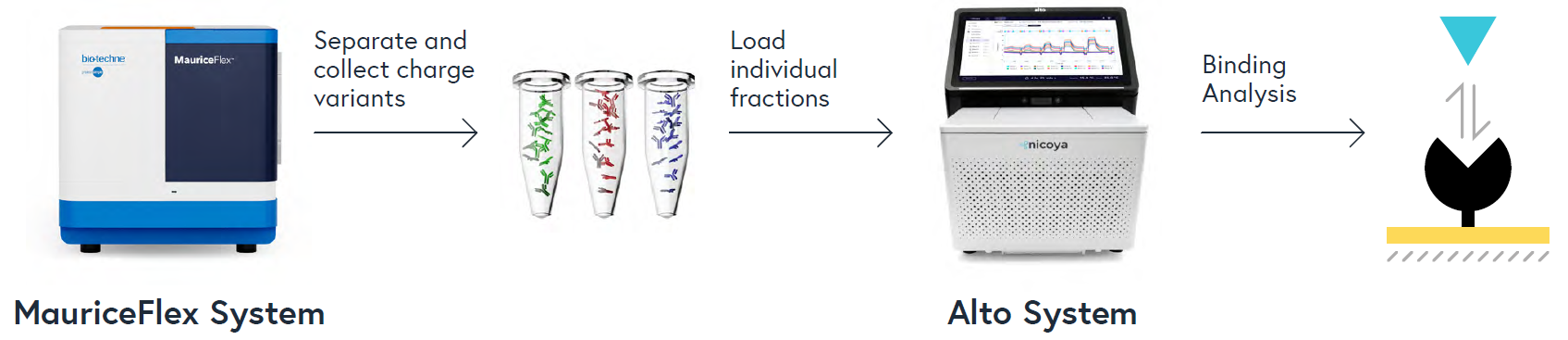
This application note presents a novel and efficient method for determining binding kinetics and affinity based on the charge heterogeneity of biological molecules. The study examined a therapeutic bispecific antibody (BsAb) called Mosunetuzumab and a research-grade biosimilar. First, the charge heterogeneity of both molecules was analyzed using imaged capillary isoelectric focusing (icIEF) on the MauriceFlex™ system, followed by the collection of individual charge variant fractions on the same system. Next, the collected fractions were tested for their ability to bind to ligands CD3 and CD20, which are the intended targets of Mosunetuzumab. To accomplish this, surface plasmon resonance (SPR) with the Alto™ System was used to measure the binding of each fraction and ligand, requiring only 2 μL of each, a key advantage over other SPR platforms due to its low sample volume requirements and higher throughput for fractionated samples. The binding data obtained from SPR correlated well with the structural information obtained from LC-MS analysis of the charge variant fractions, revealing a significantly weaker binding of the acidic fraction of the biosimilar to CD20. Overall, the workflow demonstrated in this study offers a straightforward and broadly applicable approach for correlating the charge heterogeneity of a biomolecule to its binding properties. This approach will help identify critical charge species that impact binding potency to inform the control steps needed in the process development and manufacturing of a biotherapeutic.