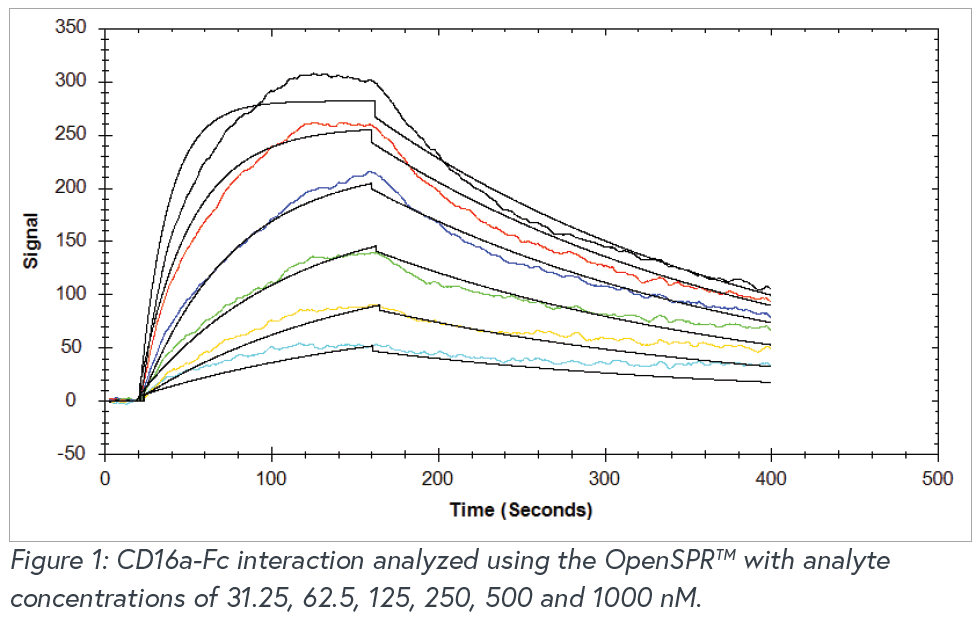
Overview
The OpenSPR™ is a powerful instrument providing researchers with in-depth label-free binding kinetics for a variety of different molecular interactions. A side by side comparison study was performed with OpenSPR™, a standard SPR instrument, and a commonly-used BLI instrument to demonstrate that OpenSPR™ can provide the same high-quality data as commonly-used instruments on the market. The standard SPR and BLI instruments are used for analyzing binding kinetics, but cost hundreds of thousands of dollars, making them inaccessible to many researchers who need this data. An Fc-FcR interaction was analyzed using the three instruments under similar conditions to show that the OpenSPR™ can generate comparable results to both the standard SPR and commonly-used BLI systems for a fraction of the cost
