Overview
Rapid changes in influenza antiviral target proteins due to antigenic drift result in cloaking of the influenza virus from the immune system of vaccinated hosts. Hence, annual formulation updates for influenza vaccines and related antibody therapies are required to preserve immune recognition against different influenza subtypes. As such, characterizing the binding kinetics and epitope diversity of various antibodies to influenza viral antigens is essential for treating and preventing potential outbreaks.
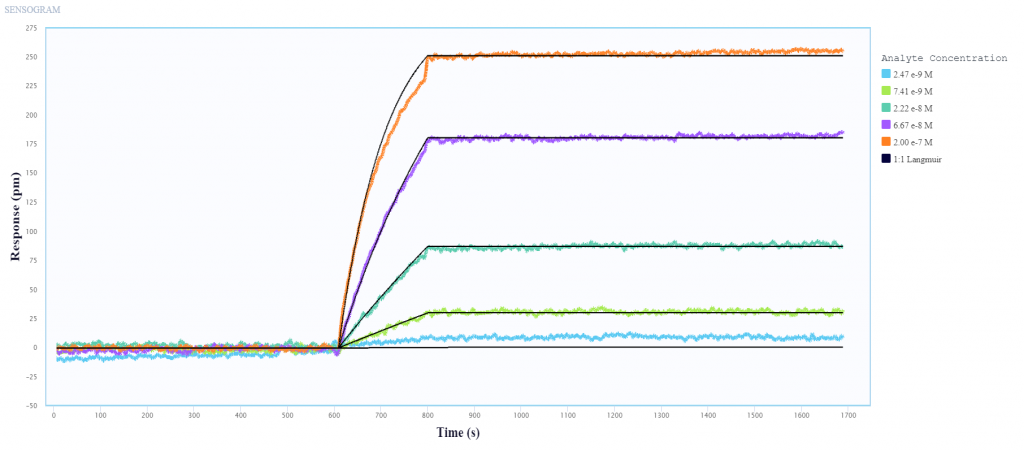
In collaboration with Sino Biological, a global leader in recombinant technology, we use Nicoya’s Alto digital surface plasmon resonance (SPR) system to perform kinetic analysis and epitope characterization of several antibodies against Influenza A nucleoprotein (NP) and hemagglutinin (HA), using only 2 µL per sample.
